 Found 17 hits with Last Name = 'barahagar' and Initial = 'ss'
Found 17 hits with Last Name = 'barahagar' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM200372
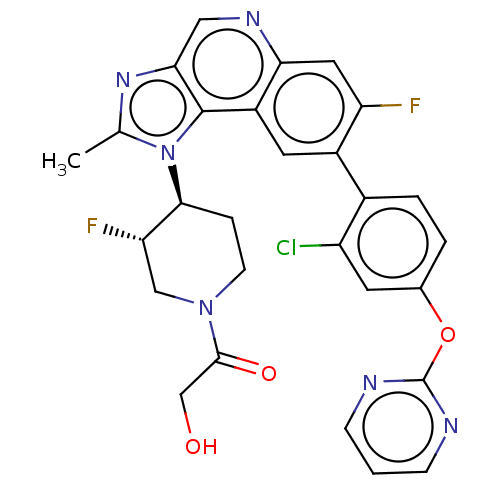
(US10011599, Example 1A | US9227969, 1A | US9629836...)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CCN(C[C@@H]1F)C(=O)CO)-c1ccc(Oc2ncccn2)cc1Cl |r| Show InChI InChI=1S/C28H23ClF2N6O3/c1-15-35-24-12-34-23-11-21(30)18(17-4-3-16(9-20(17)29)40-28-32-6-2-7-33-28)10-19(23)27(24)37(15)25-5-8-36(13-22(25)31)26(39)14-38/h2-4,6-7,9-12,22,25,38H,5,8,13-14H2,1H3/t22-,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type human partial length TRKA (G475 to G790 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50585940
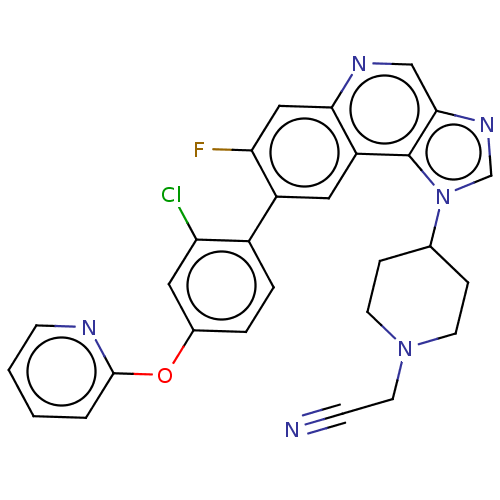
(CHEMBL5090765)Show SMILES Fc1cc2ncc3ncn(C4CCN(CC#N)CC4)c3c2cc1-c1ccc(Oc2ccccn2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human MEK1 by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50585939
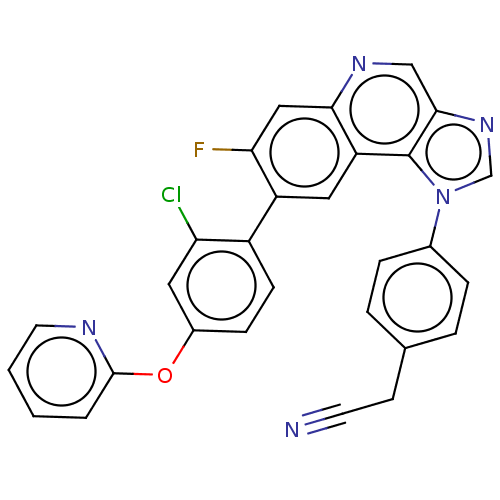
(CHEMBL5094993)Show SMILES Fc1cc2ncc3ncn(-c4ccc(CC#N)cc4)c3c2cc1-c1ccc(Oc2ccccn2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human MEK1 by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585939

(CHEMBL5094993)Show SMILES Fc1cc2ncc3ncn(-c4ccc(CC#N)cc4)c3c2cc1-c1ccc(Oc2ccccn2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585942
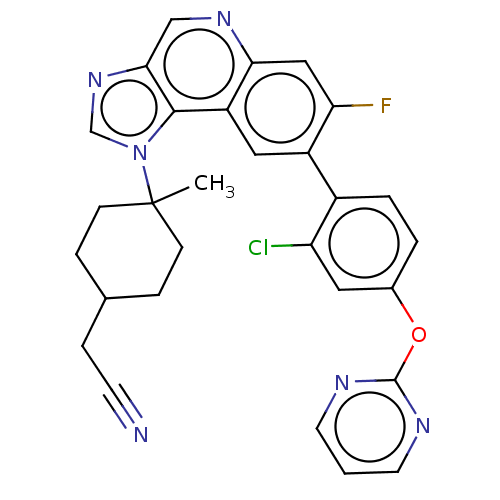
(CHEMBL5092411)Show SMILES CC1(CCC(CC#N)CC1)n1cnc2cnc3cc(F)c(cc3c12)-c1ccc(Oc2ncccn2)cc1Cl |(-4.25,.28,;-4.65,-1.21,;-3.17,-.81,;-2.08,-1.9,;-2.48,-3.38,;-1.39,-4.47,;.1,-4.08,;1.59,-3.68,;-3.96,-3.78,;-5.05,-2.69,;-5.74,-.12,;-7.27,-.28,;-7.9,1.13,;-6.76,2.16,;-6.76,3.69,;-5.43,4.47,;-4.09,3.7,;-2.77,4.47,;-1.43,3.7,;-.1,4.47,;-1.43,2.16,;-2.76,1.39,;-4.09,2.16,;-5.42,1.39,;-.1,1.39,;-.1,-.16,;1.24,-.92,;2.57,-.15,;3.9,-.92,;5.23,-.15,;5.23,1.39,;6.56,2.15,;7.9,1.38,;7.9,-.15,;6.57,-.93,;2.57,1.39,;1.24,2.16,;1.24,3.7,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585943
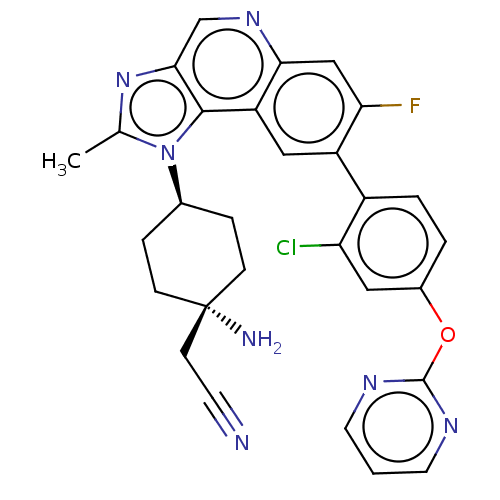
(CHEMBL5076921)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CC[C@@](N)(CC#N)CC1)-c1ccc(Oc2ncccn2)cc1Cl |r,wU:15.17,wD:18.21,(-7.97,-1.41,;-7.2,-.08,;-7.83,1.33,;-6.69,2.36,;-6.69,3.89,;-5.36,4.67,;-4.02,3.9,;-2.69,4.66,;-1.36,3.9,;-.02,4.67,;-1.36,2.36,;-2.69,1.59,;-4.02,2.36,;-5.35,1.59,;-5.67,.08,;-4.58,-1.01,;-3.09,-.61,;-2.01,-1.7,;-2.4,-3.19,;-2.8,-4.67,;-1.32,-4.27,;.17,-3.88,;1.66,-3.48,;-3.89,-3.58,;-4.98,-2.49,;-.02,1.59,;-.02,.04,;1.31,-.72,;2.64,.05,;3.97,-.72,;5.31,.05,;5.31,1.59,;6.64,2.35,;7.97,1.58,;7.97,.05,;6.64,-.73,;2.64,1.59,;1.31,2.36,;1.31,3.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585941
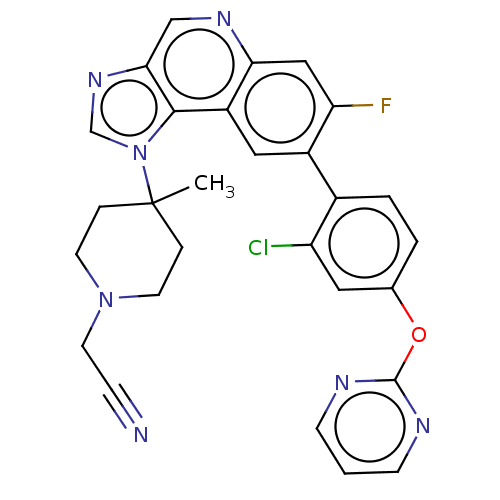
(CHEMBL5089865)Show SMILES CC1(CCN(CC#N)CC1)n1cnc2cnc3cc(F)c(cc3c12)-c1ccc(Oc2ncccn2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200455
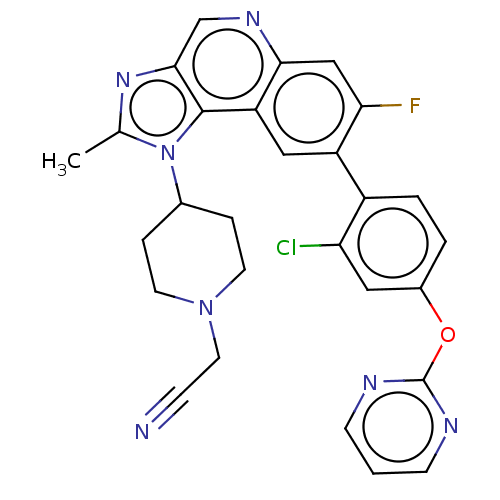
(US10011599, Example 84 | US9227969, 84)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1C1CCN(CC#N)CC1)-c1ccc(Oc2ncccn2)cc1Cl Show InChI InChI=1S/C28H23ClFN7O/c1-17-35-26-16-34-25-15-24(30)21(20-4-3-19(13-23(20)29)38-28-32-8-2-9-33-28)14-22(25)27(26)37(17)18-5-10-36(11-6-18)12-7-31/h2-4,8-9,13-16,18H,5-6,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200439
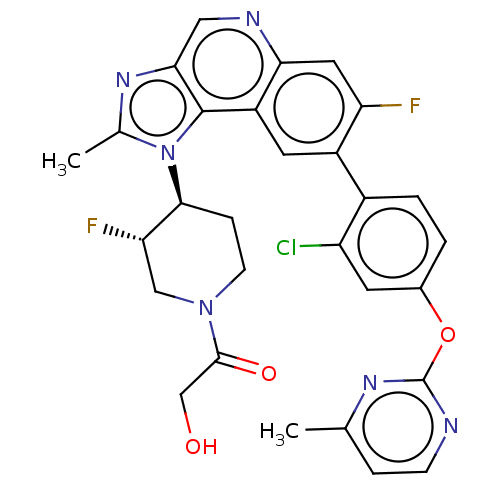
(US10011599, Example 68 | US9227969, 68 | US9629836...)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CCN(C[C@@H]1F)C(=O)CO)-c1ccc(Oc2nccc(C)n2)cc1Cl |r| Show InChI InChI=1S/C29H25ClF2N6O3/c1-15-5-7-33-29(35-15)41-17-3-4-18(21(30)9-17)19-10-20-24(11-22(19)31)34-12-25-28(20)38(16(2)36-25)26-6-8-37(13-23(26)32)27(40)14-39/h3-5,7,9-12,23,26,39H,6,8,13-14H2,1-2H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50585938
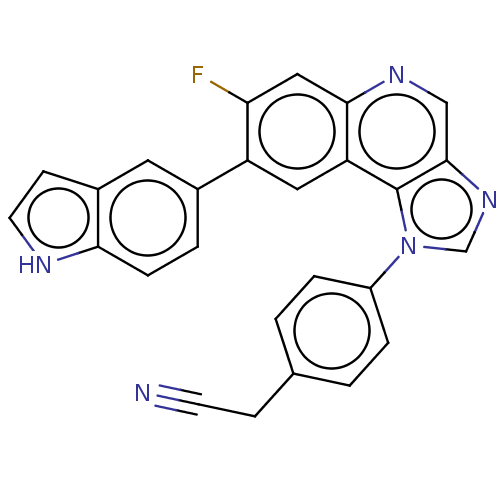
(CHEMBL5087942)Show SMILES Fc1cc2ncc3ncn(-c4ccc(CC#N)cc4)c3c2cc1-c1ccc2[nH]ccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full-length human MEK1 by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200425
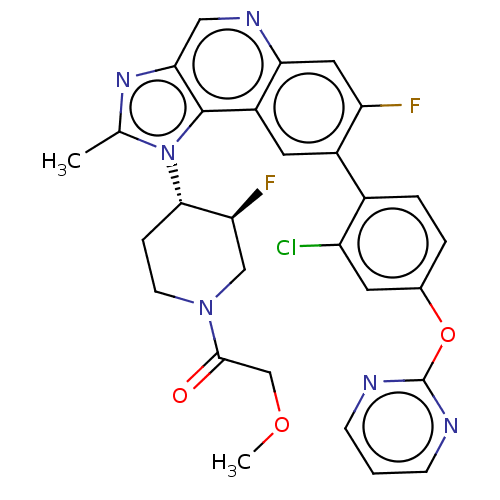
(US10011599, Example 52 | US9227969, 52)Show SMILES COCC(=O)N1CC[C@@H]([C@@H](F)C1)n1c(C)nc2cnc3cc(F)c(cc3c12)-c1ccc(Oc2ncccn2)cc1Cl |r| Show InChI InChI=1S/C29H25ClF2N6O3/c1-16-36-25-13-35-24-12-22(31)19(18-5-4-17(10-21(18)30)41-29-33-7-3-8-34-29)11-20(24)28(25)38(16)26-6-9-37(14-23(26)32)27(39)15-40-2/h3-5,7-8,10-13,23,26H,6,9,14-15H2,1-2H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585945
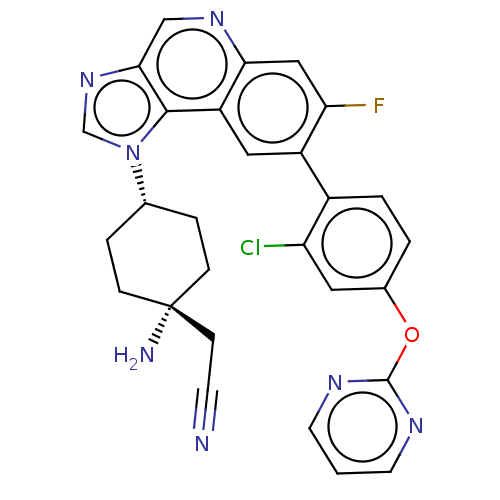
(CHEMBL5088062)Show SMILES N[C@]1(CC#N)CC[C@@H](CC1)n1cnc2cnc3cc(F)c(cc3c12)-c1ccc(Oc2ncccn2)cc1Cl |r,wU:7.10,1.0,(-2.87,-4.67,;-2.48,-3.19,;-1.39,-4.27,;.1,-3.88,;1.59,-3.48,;-2.08,-1.7,;-3.17,-.61,;-4.65,-1.01,;-5.05,-2.5,;-3.96,-3.58,;-5.74,.08,;-7.28,-.08,;-7.9,1.33,;-6.76,2.36,;-6.76,3.89,;-5.43,4.67,;-4.09,3.9,;-2.77,4.66,;-1.43,3.9,;-.1,4.67,;-1.43,2.36,;-2.76,1.59,;-4.09,2.36,;-5.42,1.59,;-.1,1.59,;-.1,.04,;1.24,-.72,;2.57,.05,;3.9,-.72,;5.23,.05,;5.23,1.59,;6.56,2.35,;7.9,1.58,;7.9,.05,;6.57,-.73,;2.57,1.59,;1.24,2.36,;1.24,3.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200372

(US10011599, Example 1A | US9227969, 1A | US9629836...)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CCN(C[C@@H]1F)C(=O)CO)-c1ccc(Oc2ncccn2)cc1Cl |r| Show InChI InChI=1S/C28H23ClF2N6O3/c1-15-35-24-12-34-23-11-21(30)18(17-4-3-16(9-20(17)29)40-28-32-6-2-7-33-28)10-19(23)27(24)37(15)25-5-8-36(13-22(25)31)26(39)14-38/h2-4,6-7,9-12,22,25,38H,5,8,13-14H2,1H3/t22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200386
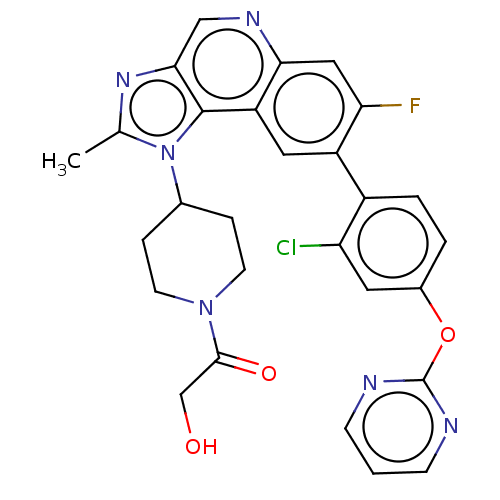
(US10011599, Example 14 | US9227969, 14 | US9629836...)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1C1CCN(CC1)C(=O)CO)-c1ccc(Oc2ncccn2)cc1Cl Show InChI InChI=1S/C28H24ClFN6O3/c1-16-34-25-14-33-24-13-23(30)20(19-4-3-18(11-22(19)29)39-28-31-7-2-8-32-28)12-21(24)27(25)36(16)17-5-9-35(10-6-17)26(38)15-37/h2-4,7-8,11-14,17,37H,5-6,9-10,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585944
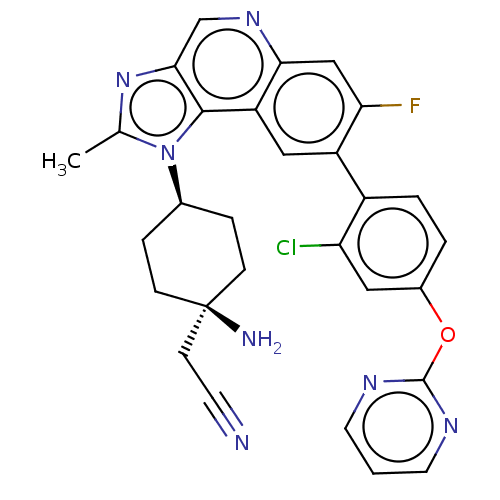
(CHEMBL5086884)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CC[C@](N)(CC#N)CC1)-c1ccc(Oc2ncccn2)cc1Cl |r,wU:15.17,18.21,(-7.97,-1.41,;-7.2,-.08,;-7.83,1.33,;-6.69,2.36,;-6.69,3.89,;-5.36,4.67,;-4.02,3.9,;-2.69,4.66,;-1.36,3.9,;-.02,4.67,;-1.36,2.36,;-2.69,1.59,;-4.02,2.36,;-5.35,1.59,;-5.67,.08,;-4.58,-1.01,;-3.09,-.61,;-2.01,-1.7,;-2.4,-3.19,;-2.8,-4.67,;-1.32,-4.27,;.17,-3.88,;1.66,-3.48,;-3.89,-3.58,;-4.98,-2.5,;-.02,1.59,;-.02,.04,;1.31,-.72,;2.64,.05,;3.97,-.72,;5.31,.05,;5.31,1.59,;6.64,2.35,;7.97,1.58,;7.97,.05,;6.65,-.73,;2.64,1.59,;1.31,2.36,;1.31,3.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM200461
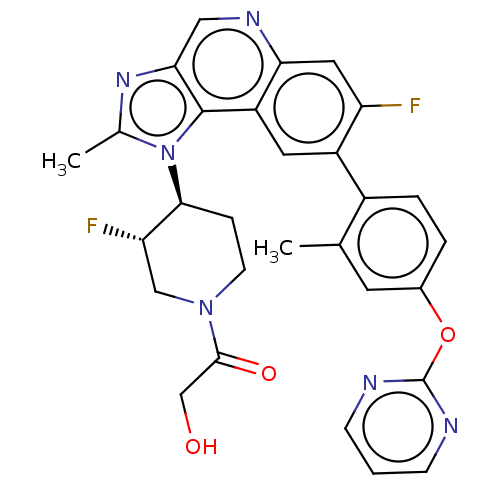
(US10011599, Example 90 | US9227969, 90 | US9629836...)Show SMILES Cc1nc2cnc3cc(F)c(cc3c2n1[C@H]1CCN(C[C@@H]1F)C(=O)CO)-c1ccc(Oc2ncccn2)cc1C |r| Show InChI InChI=1S/C29H26F2N6O3/c1-16-10-18(40-29-32-7-3-8-33-29)4-5-19(16)20-11-21-24(12-22(20)30)34-13-25-28(21)37(17(2)35-25)26-6-9-36(14-23(26)31)27(39)15-38/h3-5,7-8,10-13,23,26,38H,6,9,14-15H2,1-2H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585946

(CHEMBL5085569)Show SMILES OCC(=O)N1CC[C@@H]([C@@H](F)C1)n1cnc2cnc3cc(F)c(cc3c12)-c1ccc(Oc2ncccn2)cc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02192
BindingDB Entry DOI: 10.7270/Q26Q225J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data