

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50471134 (CHEMBL64664) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50506044 (CHEMBL4531537) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080882 (Benzamidrazone analogue | CHEMBL312244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080879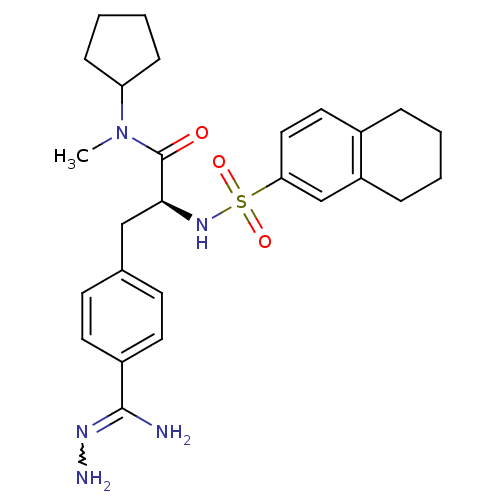 (Benzamidrazone analogue | CHEMBL82057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50506042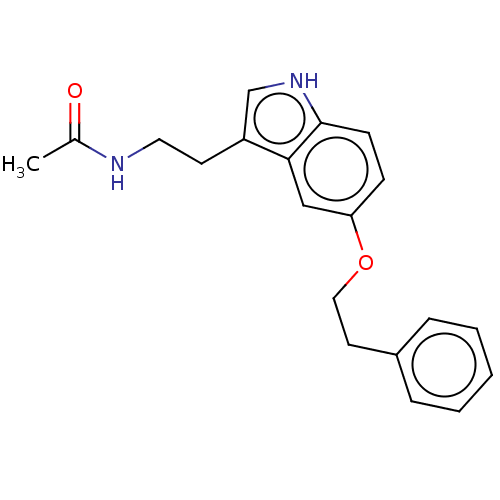 (CHEMBL4467954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 1 hr by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in COS7 cells incubated for 1.5 hrs by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Binding affinity to MT2 receptor (unknown origin) assessed as inhibition constant | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50506042 (CHEMBL4467954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 1 hr by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50043289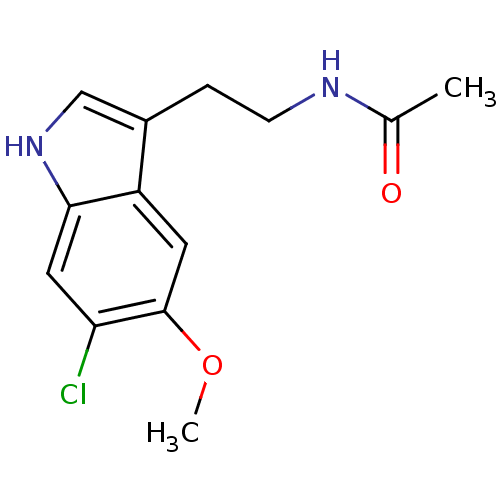 (CHEMBL34730 | N-[2-(6-Chloro-5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Binding affinity to MT2 receptor (unknown origin) assessed as inhibition constant | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50086029 (CHEMBL10099 | N-[2-(10-Methoxy-5,6-dihydro-indolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in NIH3T3 cells membranes by radioligand binding assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080885 (Benzamidrazone analogue | CHEMBL79304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080883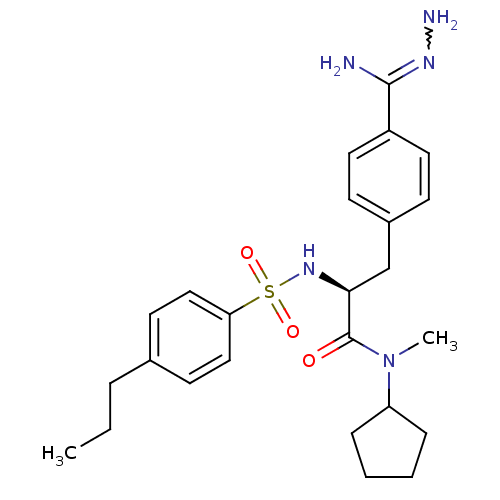 (Benzamidrazone analogue | CHEMBL310664) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in NIH3T3 cells membranes incubated for 90 mins by Cheng-Prusoff equation an... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50506045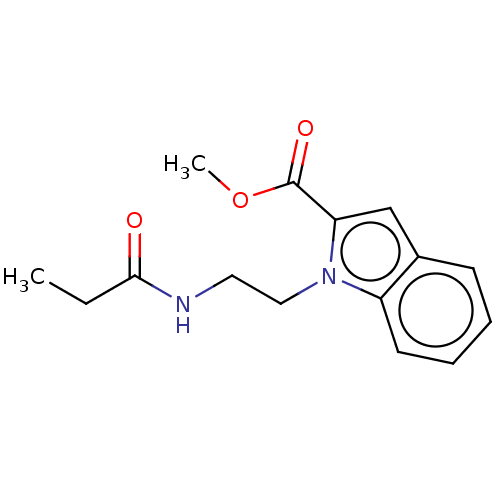 (CHEMBL4475742) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM85066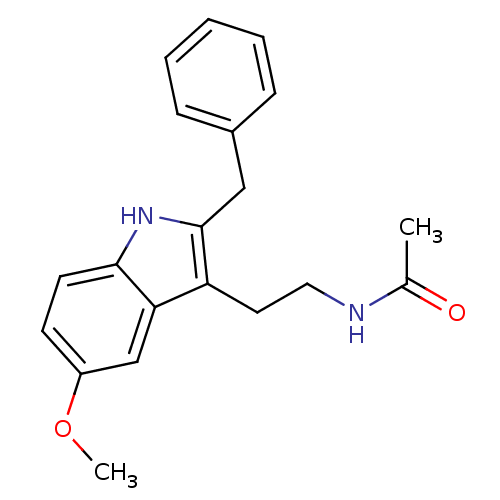 (Luzindole,5-Methoxy) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in COS7 cells incubated for 1.5 hrs by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in NIH3T3 cells membranes incubated for 90 mins by Cheng-Prusoff equation an... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in NIH3T3 cells membranes by radioligand binding assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472142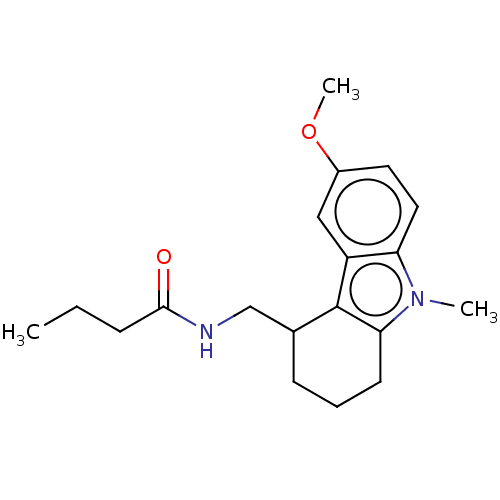 (CHEMBL142343) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from chick brain melatonin receptor | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085048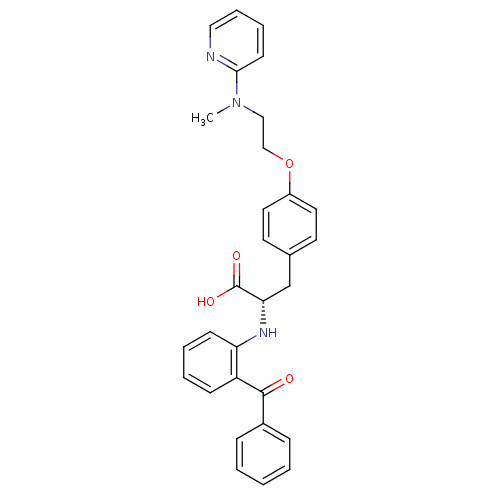 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PPARgamma (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.028 BindingDB Entry DOI: 10.7270/Q2930XSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 1 hr by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070780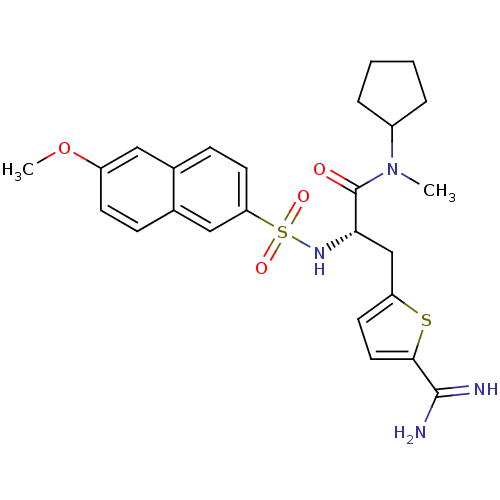 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080888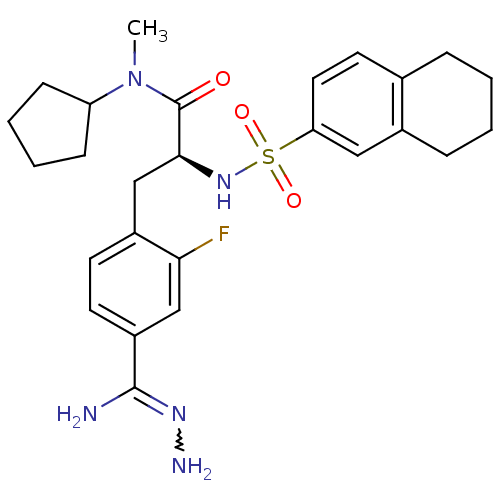 (Benzamidrazone analogue | CHEMBL84454) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from chick brain melatonin receptor | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070785 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in NIH3T3 cells membranes by radioligand binding assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080886 (Benzamidrazone analogue | CHEMBL313296) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661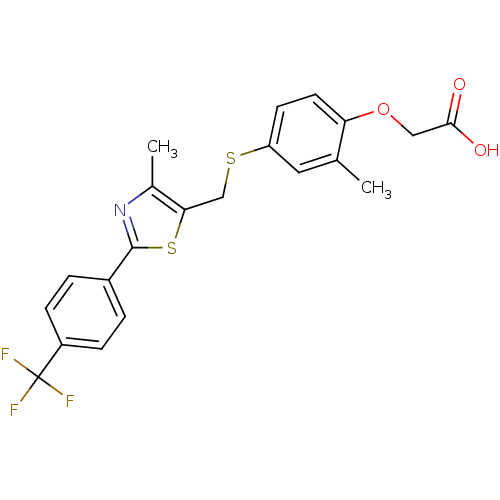 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PPARdelta (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.028 BindingDB Entry DOI: 10.7270/Q2930XSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070782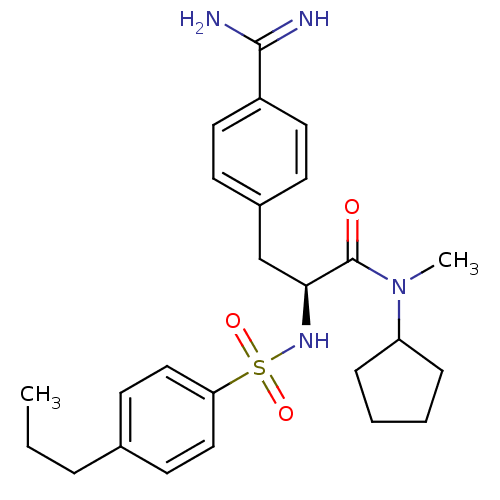 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in COS7 cells incubated for 1.5 hrs by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Binding affinity to MT1 receptor (unknown origin) assessed as inhibition constant | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080884 (Benzamidrazone analogue | CHEMBL314189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT2 receptor expressed in CHO cells incubated for 1 hr by gamma counting method | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50291355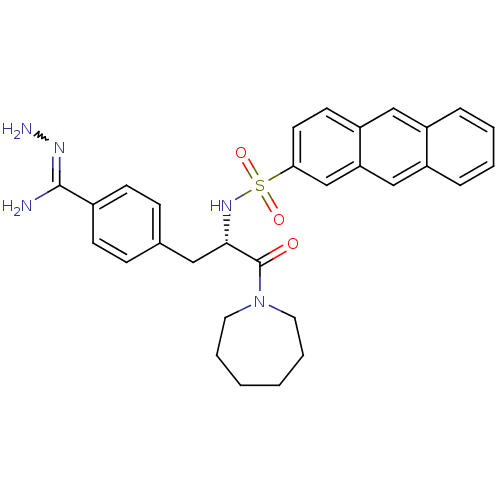 (1-[3-[4-amino(amineimino)methylphenyl]-2-(2-anthry...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory effect against bovine thrombin | Bioorg Med Chem Lett 7: 769-774 (1997) Article DOI: 10.1016/S0960-894X(97)00115-7 BindingDB Entry DOI: 10.7270/Q2B27V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085048 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085048 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2021.116564 BindingDB Entry DOI: 10.7270/Q29G5RSJ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491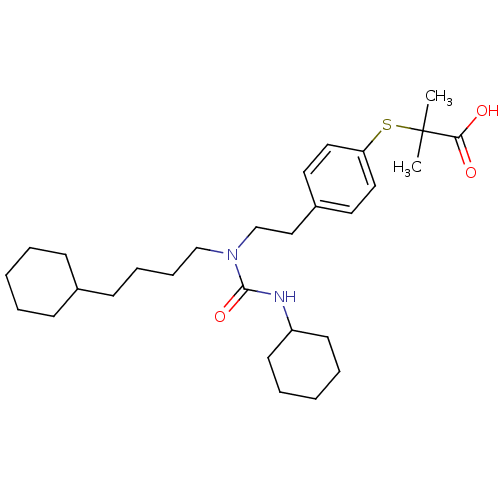 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PPARalpha (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2019.05.028 BindingDB Entry DOI: 10.7270/Q2930XSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50506043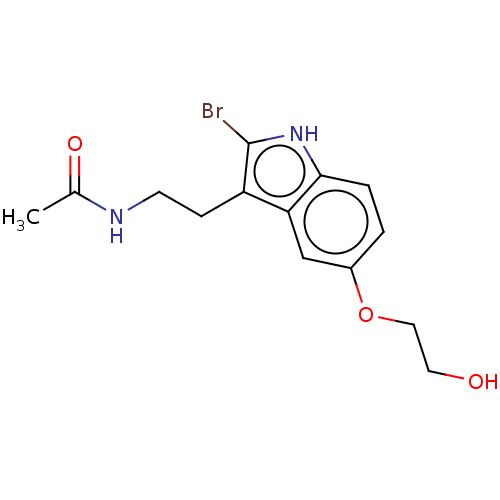 (CHEMBL4548591) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in NIH3T3 cells membranes incubated for 90 mins by Cheng-Prusoff equation an... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069297 (1-[3-[4-amino(amineimino)methylphenyl]-2-(2-naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory effect against bovine thrombin | Bioorg Med Chem Lett 7: 769-774 (1997) Article DOI: 10.1016/S0960-894X(97)00115-7 BindingDB Entry DOI: 10.7270/Q2B27V9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080880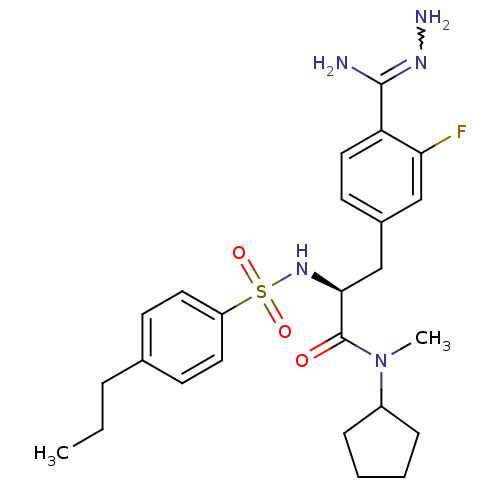 (Benzamidrazone analogue | CHEMBL312011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080881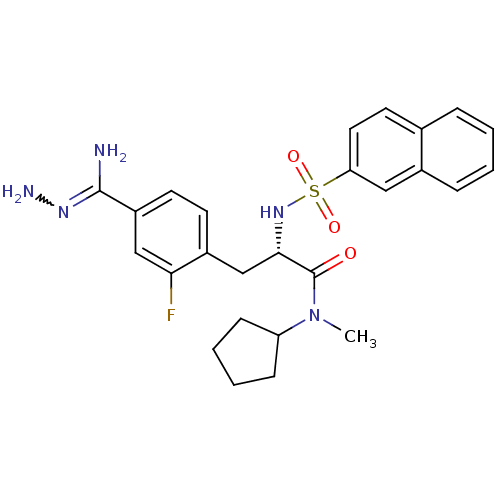 (Benzamidrazone analogue | CHEMBL82072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070784 ((S)-3-(5-Carbamimidoyl-thiophen-2-yl)-N-cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1683-6 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069292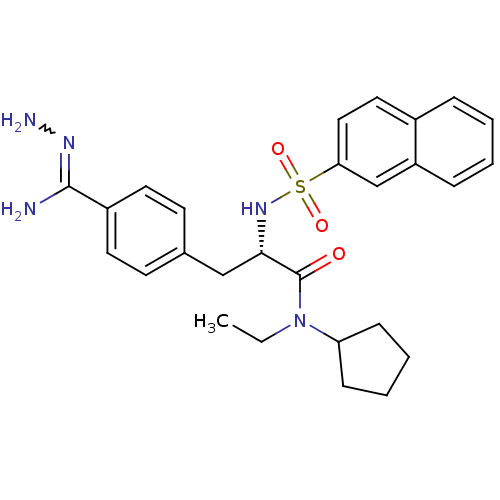 (CHEMBL156082 | N-ethyl-N-cyclopentyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50086029 (CHEMBL10099 | N-[2-(10-Methoxy-5,6-dihydro-indolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nantong University Curated by ChEMBL | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in NIH3T3 cells membranes by radioligand binding assay | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111847 BindingDB Entry DOI: 10.7270/Q2H70K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076722 ((S)-5-(4-Methylamino-phenyl)-2-(5,6,7,8-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition of human thrombin | Bioorg Med Chem Lett 9: 1013-8 (1999) BindingDB Entry DOI: 10.7270/Q298866C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1224 total ) | Next | Last >> |