 Found 717 hits with Last Name = 'fujii' and Initial = 't'
Found 717 hits with Last Name = 'fujii' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099066
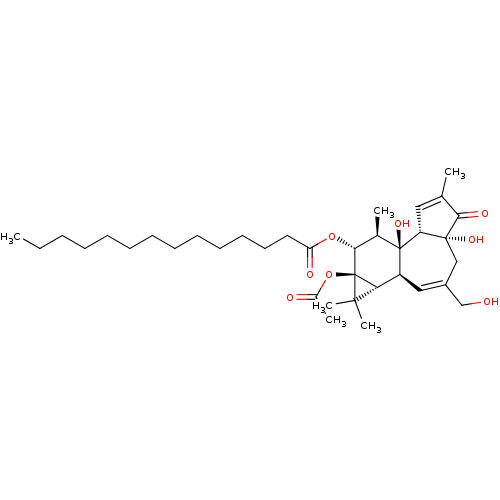
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50057509
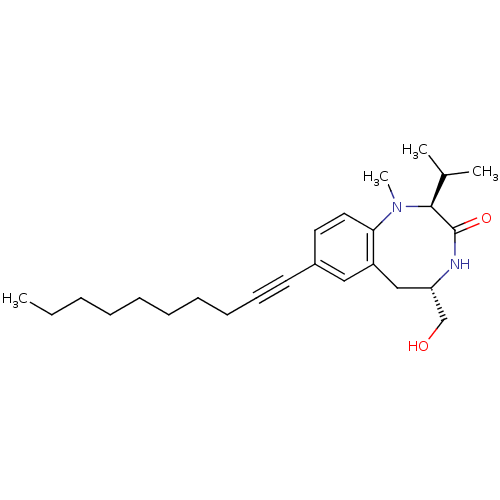
((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50057509

((2S,5S)-8-Dec-1-ynyl-5-hydroxymethyl-2-isopropyl-1...)Show SMILES CCCCCCCCC#Cc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc2c1 Show InChI InChI=1S/C25H38N2O2/c1-5-6-7-8-9-10-11-12-13-20-14-15-23-21(16-20)17-22(18-28)26-25(29)24(19(2)3)27(23)4/h14-16,19,22,24,28H,5-11,17-18H2,1-4H3,(H,26,29)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099067
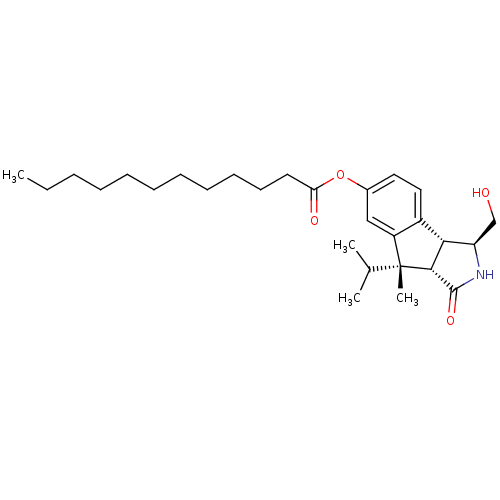
(CHEMBL173348 | Dodecanoic acid (3S,3aR,8S,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50099064
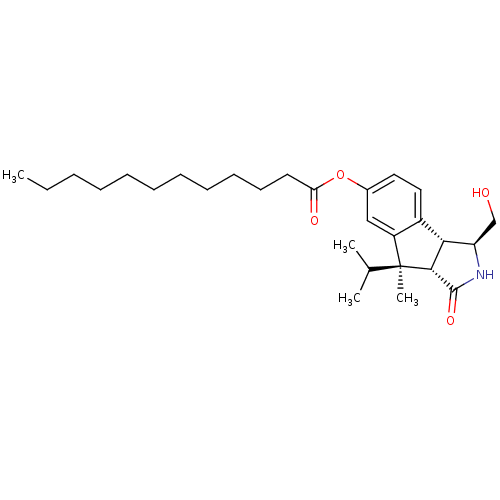
(CHEMBL368720 | Dodecanoic acid (3S,3aR,8R,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099067

(CHEMBL173348 | Dodecanoic acid (3S,3aR,8S,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50099065

((3S,3aR,8R,8aS)-6-Dec-1-ynyl-3-hydroxymethyl-8-iso...)Show SMILES CCCCCCCCC#Cc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C26H37NO2/c1-5-6-7-8-9-10-11-12-13-19-14-15-20-21(16-19)26(4,18(2)3)24-23(20)22(17-28)27-25(24)29/h14-16,18,22-24,28H,5-11,17H2,1-4H3,(H,27,29)/t22-,23-,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50099067

(CHEMBL173348 | Dodecanoic acid (3S,3aR,8S,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C gamma |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099064

(CHEMBL368720 | Dodecanoic acid (3S,3aR,8R,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099065

((3S,3aR,8R,8aS)-6-Dec-1-ynyl-3-hydroxymethyl-8-iso...)Show SMILES CCCCCCCCC#Cc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C26H37NO2/c1-5-6-7-8-9-10-11-12-13-19-14-15-20-21(16-19)26(4,18(2)3)24-23(20)22(17-28)27-25(24)29/h14-16,18,22-24,28H,5-11,17H2,1-4H3,(H,27,29)/t22-,23-,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099064

(CHEMBL368720 | Dodecanoic acid (3S,3aR,8R,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099065

((3S,3aR,8R,8aS)-6-Dec-1-ynyl-3-hydroxymethyl-8-iso...)Show SMILES CCCCCCCCC#Cc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C26H37NO2/c1-5-6-7-8-9-10-11-12-13-19-14-15-20-21(16-19)26(4,18(2)3)24-23(20)22(17-28)27-25(24)29/h14-16,18,22-24,28H,5-11,17H2,1-4H3,(H,27,29)/t22-,23-,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099064

(CHEMBL368720 | Dodecanoic acid (3S,3aR,8R,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 593 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099067

(CHEMBL173348 | Dodecanoic acid (3S,3aR,8S,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C alpha |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099065

((3S,3aR,8R,8aS)-6-Dec-1-ynyl-3-hydroxymethyl-8-iso...)Show SMILES CCCCCCCCC#Cc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C26H37NO2/c1-5-6-7-8-9-10-11-12-13-19-14-15-20-21(16-19)26(4,18(2)3)24-23(20)22(17-28)27-25(24)29/h14-16,18,22-24,28H,5-11,17H2,1-4H3,(H,27,29)/t22-,23-,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 701 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099067

(CHEMBL173348 | Dodecanoic acid (3S,3aR,8S,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099064

(CHEMBL368720 | Dodecanoic acid (3S,3aR,8R,8aS)-3-h...)Show SMILES CCCCCCCCCCCC(=O)Oc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C28H43NO4/c1-5-6-7-8-9-10-11-12-13-14-24(31)33-20-15-16-21-22(17-20)28(4,19(2)3)26-25(21)23(18-30)29-27(26)32/h15-17,19,23,25-26,30H,5-14,18H2,1-4H3,(H,29,32)/t23-,25-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099065

((3S,3aR,8R,8aS)-6-Dec-1-ynyl-3-hydroxymethyl-8-iso...)Show SMILES CCCCCCCCC#Cc1ccc2[C@@H]3[C@@H](CO)NC(=O)[C@@H]3[C@@](C)(C(C)C)c2c1 Show InChI InChI=1S/C26H37NO2/c1-5-6-7-8-9-10-11-12-13-19-14-15-20-21(16-19)26(4,18(2)3)24-23(20)22(17-28)27-25(24)29/h14-16,18,22-24,28H,5-11,17H2,1-4H3,(H,27,29)/t22-,23-,24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611197
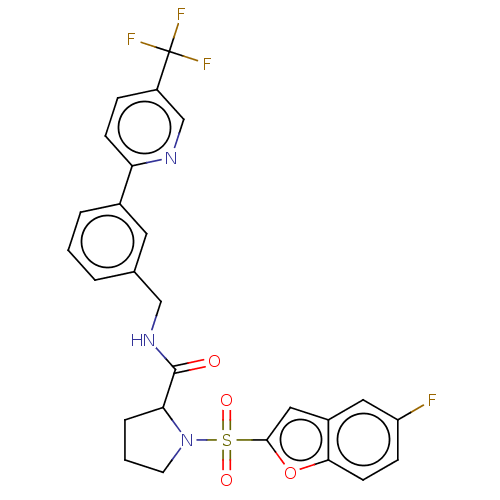
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[3-[5...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(c1)-c1ccc(cn1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50121716

((3-methyl-4-(3-(pyridin-3-ylmethylamino)propoxy)be...)Show SMILES Cc1c(oc2cccc(OCCCNCc3cccnc3)c12)C(=O)c1ccc(C)cn1 Show InChI InChI=1S/C25H25N3O3/c1-17-9-10-20(28-14-17)24(29)25-18(2)23-21(7-3-8-22(23)31-25)30-13-5-12-27-16-19-6-4-11-26-15-19/h3-4,6-11,14-15,27H,5,12-13,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) |
Bioorg Med Chem Lett 13: 87-91 (2003)
BindingDB Entry DOI: 10.7270/Q25D8V2K |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50220561

(CHEMBL173370)Show SMILES Cc1c(oc2cccc(OCCCNCc3cccnc3)c12)C(=O)c1nc2ccccc2n1C Show InChI InChI=1S/C27H26N4O3/c1-18-24-22(33-15-7-14-29-17-19-8-6-13-28-16-19)11-5-12-23(24)34-26(18)25(32)27-30-20-9-3-4-10-21(20)31(27)2/h3-6,8-13,16,29H,7,14-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) |
Bioorg Med Chem Lett 13: 87-91 (2003)
BindingDB Entry DOI: 10.7270/Q25D8V2K |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611196
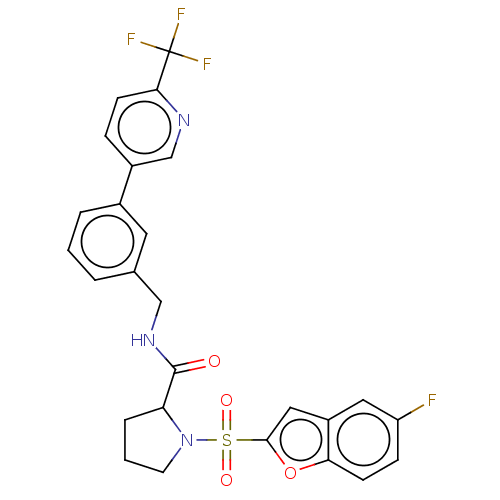
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[3-[6...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(c1)-c1ccc(nc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611264
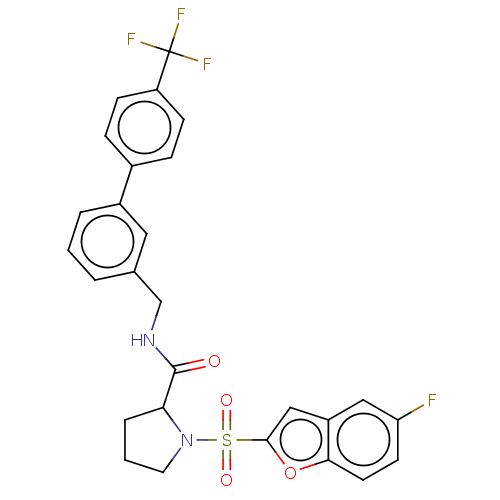
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[3-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(c1)-c1ccc(cc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50220562
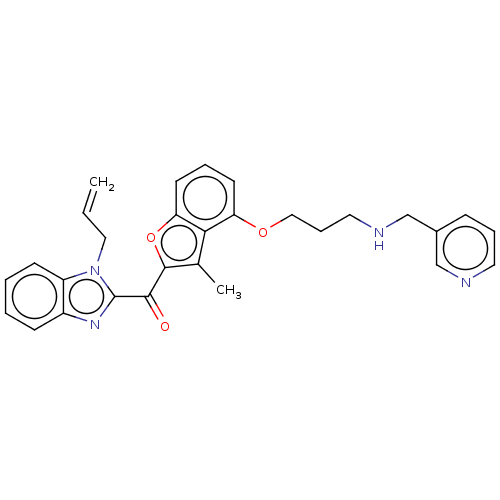
(CHEMBL171506)Show SMILES Cc1c(oc2cccc(OCCCNCc3cccnc3)c12)C(=O)c1nc2ccccc2n1CC=C Show InChI InChI=1S/C29H28N4O3/c1-3-16-33-23-11-5-4-10-22(23)32-29(33)27(34)28-20(2)26-24(12-6-13-25(26)36-28)35-17-8-15-31-19-21-9-7-14-30-18-21/h3-7,9-14,18,31H,1,8,15-17,19H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) |
Bioorg Med Chem Lett 13: 87-91 (2003)
BindingDB Entry DOI: 10.7270/Q25D8V2K |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611235
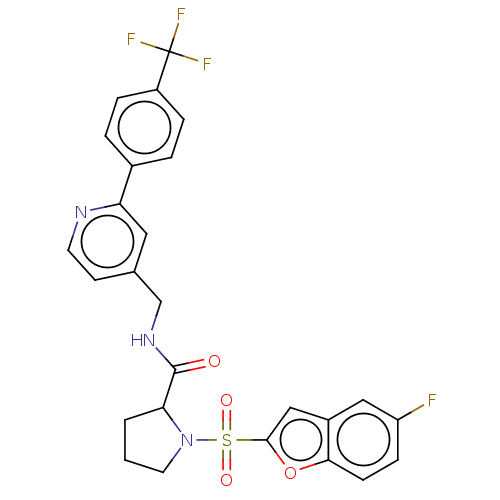
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[2-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1ccnc(c1)-c1ccc(cc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611236
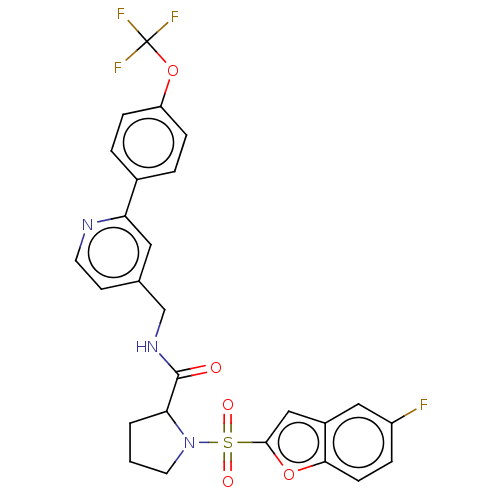
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[2-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1ccnc(c1)-c1ccc(OC(F)(F)F)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611331
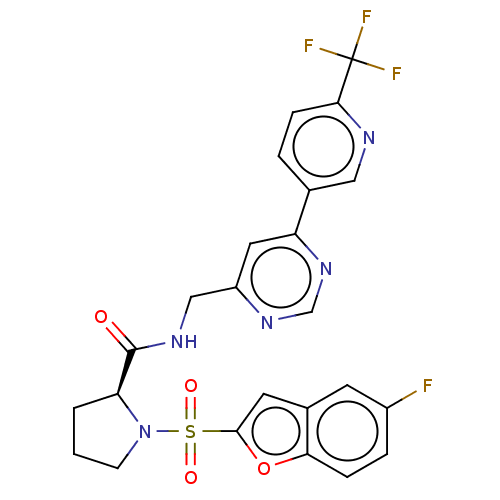
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[6-[6...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCC[C@H]1C(=O)NCc1cc(ncn1)-c1ccc(nc1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50220565
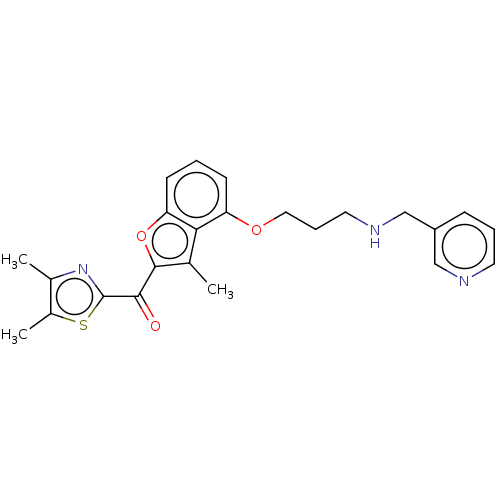
(CHEMBL170524)Show SMILES Cc1nc(sc1C)C(=O)c1oc2cccc(OCCCNCc3cccnc3)c2c1C Show InChI InChI=1S/C24H25N3O3S/c1-15-21-19(29-12-6-11-26-14-18-7-5-10-25-13-18)8-4-9-20(21)30-23(15)22(28)24-27-16(2)17(3)31-24/h4-5,7-10,13,26H,6,11-12,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) |
Bioorg Med Chem Lett 13: 87-91 (2003)
BindingDB Entry DOI: 10.7270/Q25D8V2K |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611205
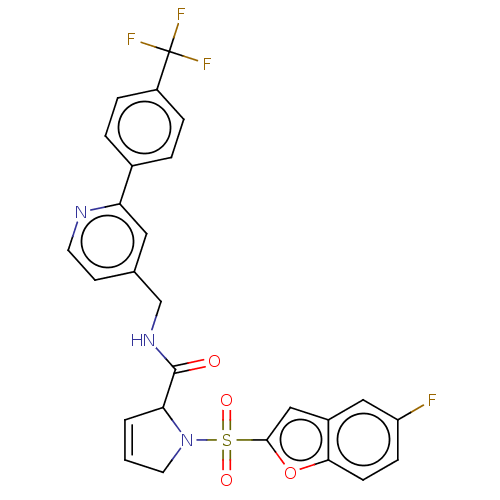
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[2-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CC=CC1C(=O)NCc1ccnc(c1)-c1ccc(cc1)C(F)(F)F |c:17| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611265
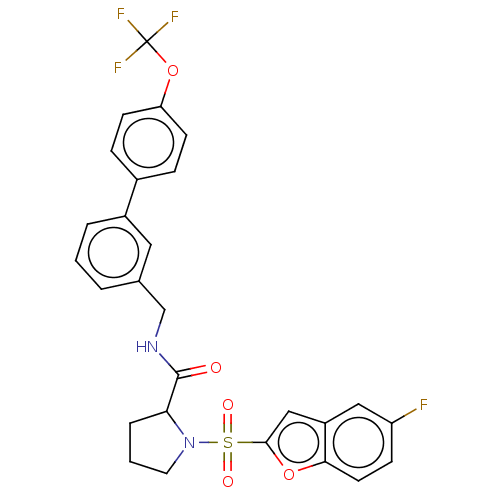
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[3-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(c1)-c1ccc(OC(F)(F)F)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611240
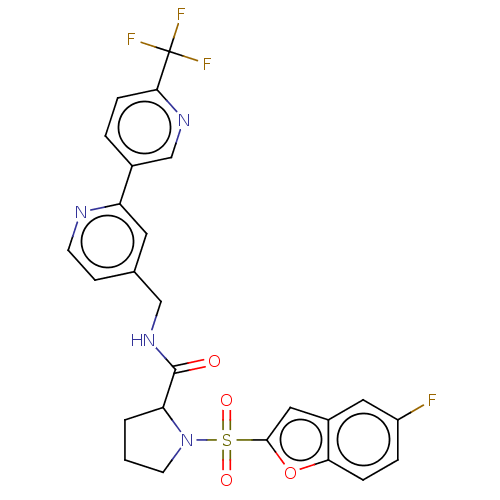
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[2-[6...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1ccnc(c1)-c1ccc(nc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611198
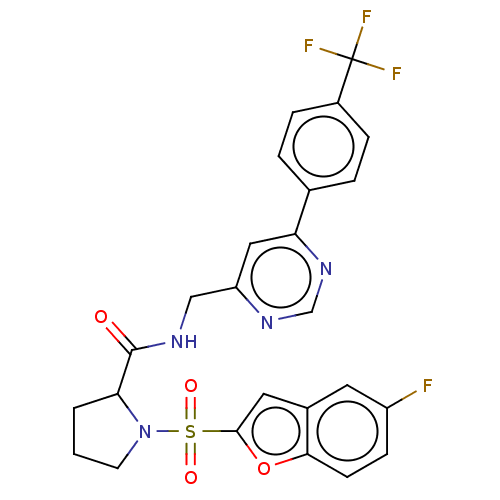
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[6-[4...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cc(ncn1)-c1ccc(cc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611330
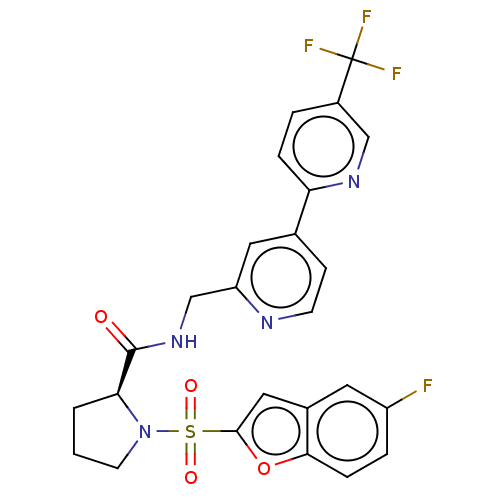
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[4-[5...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCC[C@H]1C(=O)NCc1cc(ccn1)-c1ccc(cn1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611245
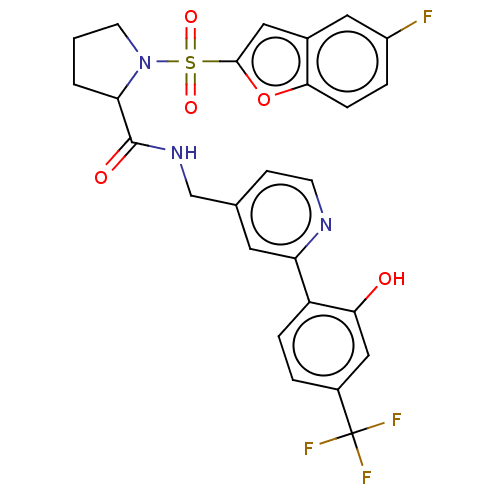
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[2-[2...)Show SMILES Oc1cc(ccc1-c1cc(CNC(=O)C2CCCN2S(=O)(=O)c2cc3cc(F)ccc3o2)ccn1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611095
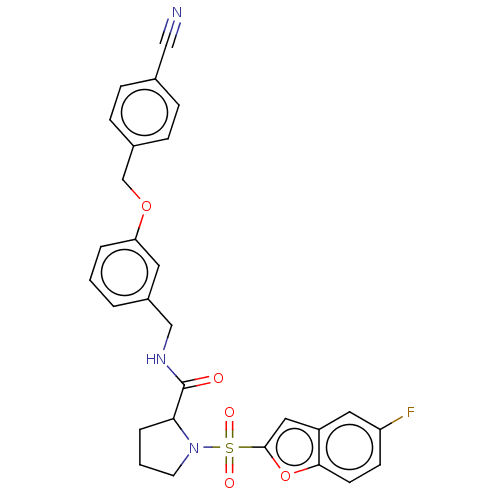
((2S)-N-[[3-[(4-cyanophenyl) methoxy]phenyl]methyl]...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(OCc2ccc(cc2)C#N)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50030174
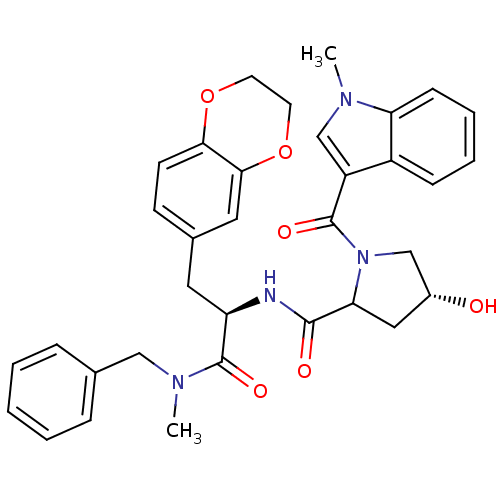
((R)-4-Hydroxy-1-(1-methyl-1H-indole-3-carbonyl)-py...)Show SMILES CN(Cc1ccccc1)C(=O)[C@@H](Cc1ccc2OCCOc2c1)NC(=O)C1C[C@@H](O)CN1C(=O)c1cn(C)c2ccccc12 Show InChI InChI=1S/C34H36N4O6/c1-36-21-26(25-10-6-7-11-28(25)36)33(41)38-20-24(39)18-29(38)32(40)35-27(34(42)37(2)19-22-8-4-3-5-9-22)16-23-12-13-30-31(17-23)44-15-14-43-30/h3-13,17,21,24,27,29,39H,14-16,18-20H2,1-2H3,(H,35,40)/t24-,27-,29?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity against substance P receptor using [3H]SP as radioligand in guinea pig lung membrane |
J Med Chem 37: 2090-9 (1994)
BindingDB Entry DOI: 10.7270/Q23779BT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611366
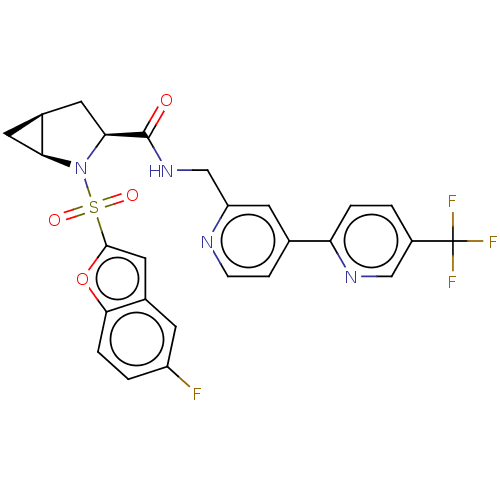
((1S,3S,5S)-4-(5- fluorobenzofuran-2-yl)sulfonyl- N...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1[C@H]2C[C@H]2C[C@H]1C(=O)NCc1cc(ccn1)-c1ccc(cn1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611193
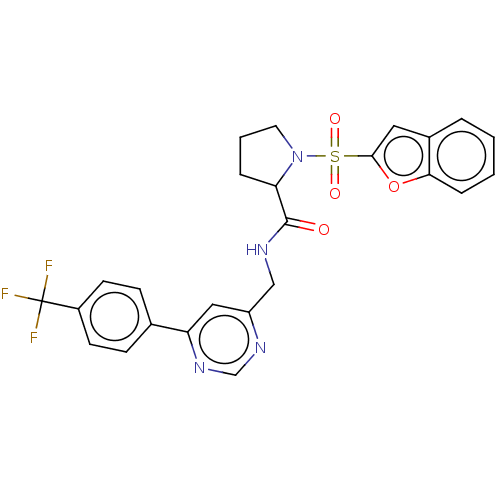
((2S)-1-(benzofuran-2-ylsulfonyl)- N-[[6-[4-(triflu...)Show SMILES FC(F)(F)c1ccc(cc1)-c1cc(CNC(=O)C2CCCN2S(=O)(=O)c2cc3ccccc3o2)ncn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611192

((2S)-1-(benzofuran-2-ylsulfonyl)- N-[[3-[5-(triflu...)Show SMILES FC(F)(F)c1ccc(nc1)-c1cccc(CNC(=O)C2CCCN2S(=O)(=O)c2cc3ccccc3o2)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611332
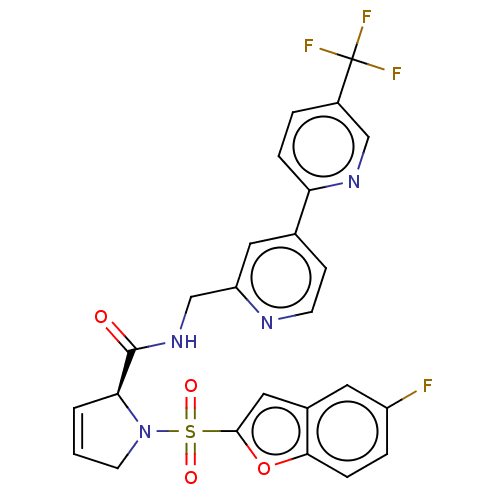
((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[4-[5...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CC=C[C@H]1C(=O)NCc1cc(ccn1)-c1ccc(cn1)C(F)(F)F |r,c:17| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50220569
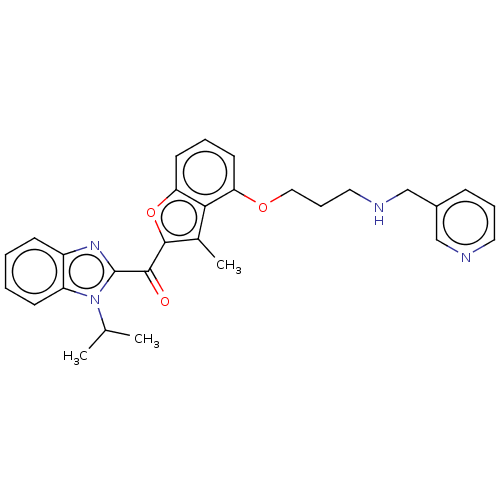
(CHEMBL422896)Show SMILES CC(C)n1c(nc2ccccc12)C(=O)c1oc2cccc(OCCCNCc3cccnc3)c2c1C Show InChI InChI=1S/C29H30N4O3/c1-19(2)33-23-11-5-4-10-22(23)32-29(33)27(34)28-20(3)26-24(12-6-13-25(26)36-28)35-16-8-15-31-18-21-9-7-14-30-17-21/h4-7,9-14,17,19,31H,8,15-16,18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) |
Bioorg Med Chem Lett 13: 87-91 (2003)
BindingDB Entry DOI: 10.7270/Q25D8V2K |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611286

((2S)-1-(benzofuran-2- ylsulfonyl)-N-[[4-[5- (trifl...)Show SMILES FC(F)(F)c1ccc(nc1)-c1ccnc(CNC(=O)C2CCCN2S(=O)(=O)c2cc3ccccc3o2)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM611154

((2S)-1-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[3-[[...)Show SMILES Fc1ccc2oc(cc2c1)S(=O)(=O)N1CCCC1C(=O)NCc1cccc(OCc2ccc(cc2)C(F)(F)F)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81J54 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data