 Found 1242 hits with Last Name = 'ho' and Initial = 'tc'
Found 1242 hits with Last Name = 'ho' and Initial = 'tc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
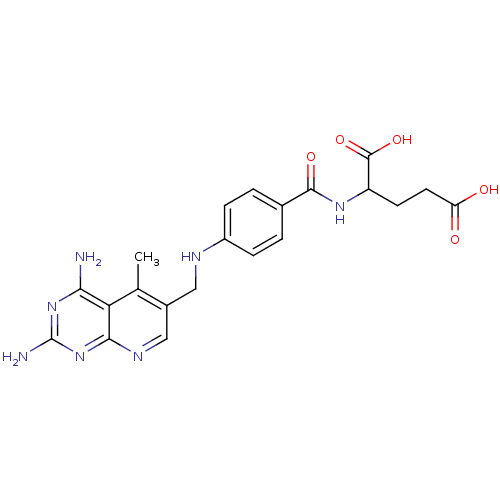
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023682

(2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2nc(N)nc(N)c12)-c1ccccc1 Show InChI InChI=1S/C27H27N7O5/c1-14-18(22(15-5-3-2-4-6-15)32-24-21(14)23(28)33-27(29)34-24)13-30-17-9-7-16(8-10-17)25(37)31-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,31,37)(H,35,36)(H,38,39)(H4,28,29,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023680

(2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2n1)-c1ccccc1 Show InChI InChI=1S/C26H25N7O5/c27-22-18-12-16(21(14-4-2-1-3-5-14)31-23(18)33-26(28)32-22)13-29-17-8-6-15(7-9-17)24(36)30-19(25(37)38)10-11-20(34)35/h1-9,12,19,29H,10-11,13H2,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023683
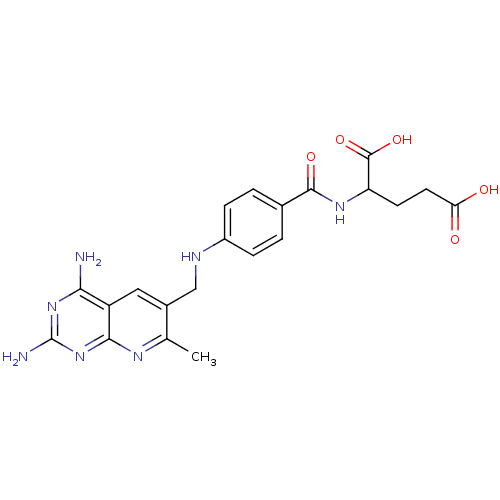
(2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1nc2nc(N)nc(N)c2cc1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10-12(8-14-17(22)27-21(23)28-18(14)25-10)9-24-13-4-2-11(3-5-13)19(31)26-15(20(32)33)6-7-16(29)30/h2-5,8,15,24H,6-7,9H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023684
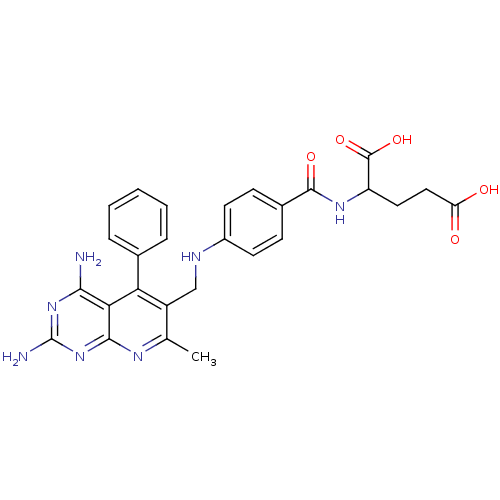
(2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...)Show SMILES Cc1nc2nc(N)nc(N)c2c(-c2ccccc2)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C27H27N7O5/c1-14-18(21(15-5-3-2-4-6-15)22-23(28)33-27(29)34-24(22)31-14)13-30-17-9-7-16(8-10-17)25(37)32-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,32,37)(H,35,36)(H,38,39)(H4,28,29,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Pyruvate decarboxylase
(Zymomonas mobilis subsp. mobilis (strain ATCC 3182...) | BDBM50589544
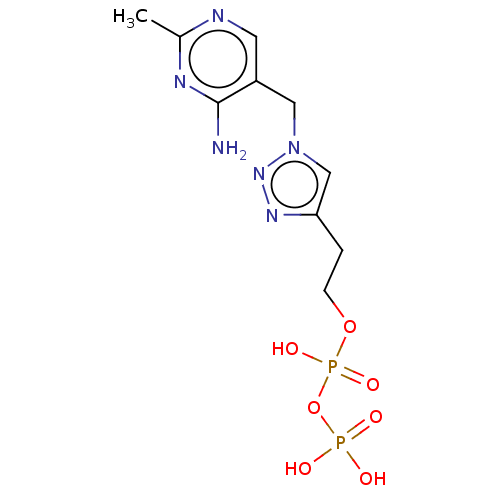
(CHEMBL3559521)Show SMILES Cc1ncc(Cn2cc(CCOP(O)(=O)OP(O)(O)=O)nn2)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00085g
BindingDB Entry DOI: 10.7270/Q2M90DMB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699
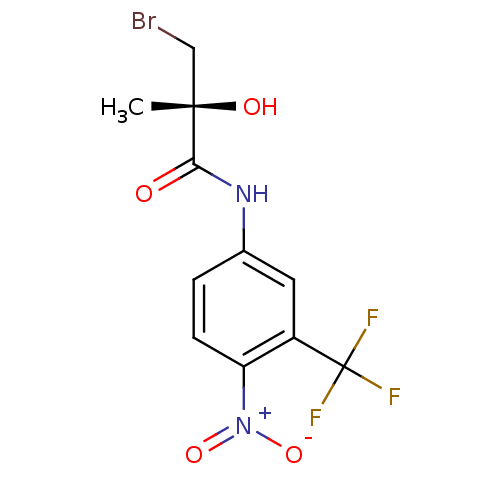
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM50557516
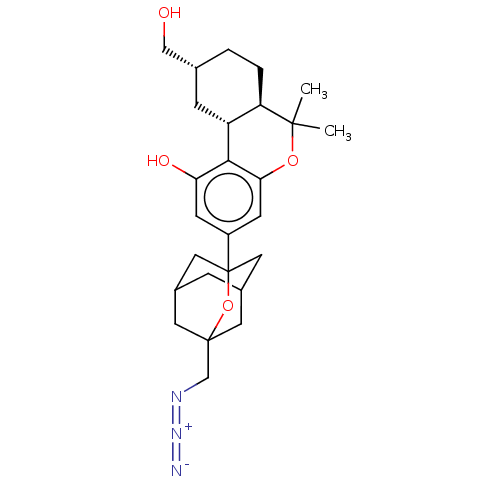
(CHEMBL4787347)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50557512
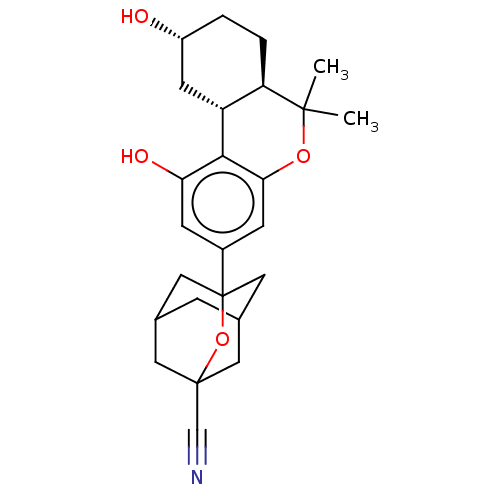
(CHEMBL4779775)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C#N)C2 |r,TLB:23:24:21.22.27:28,29:26:21:31.23.24,THB:23:22:28:31.24.25,25:24:21:27.26.28,25:26:21:31.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557516

(CHEMBL4787347)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557516

(CHEMBL4787347)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557513

(CHEMBL4795739)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50557513

(CHEMBL4795739)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557513

(CHEMBL4795739)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=[N+]=[N-])(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557514
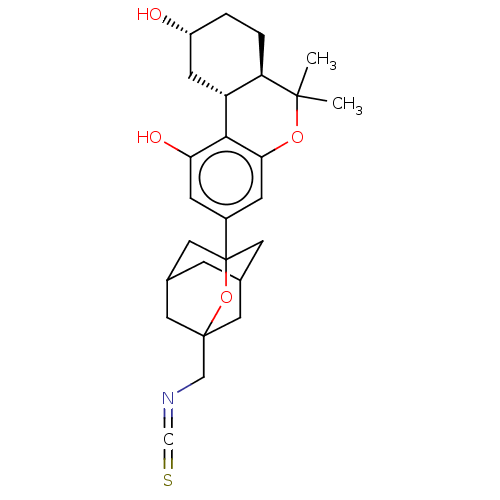
(CHEMBL4779296)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557517
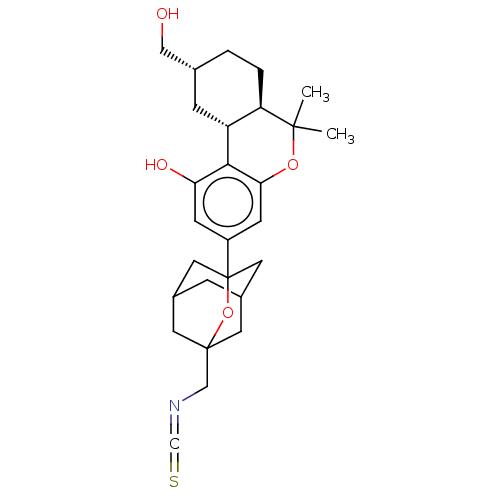
(CHEMBL4760082)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557514

(CHEMBL4779296)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG/His-tagged HDAC1 (1 to 482 residues) expressed in sf9 cells using acetyl-Lys(Ac)-AMC as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM50557517

(CHEMBL4760082)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human C-terminal His-tagged HDAC3 (1 to 428 residues)/human N-terminal GST-tagged NcoR2 (395 to 489 residues) expressed in sf9 cells us... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557517

(CHEMBL4760082)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:24:25:22.23.32:33,28:27:22:34.24.25,THB:24:23:33:34.25.26,26:25:22:32.27.33,26:27:22:34.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50557514

(CHEMBL4779296)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(CN=C=S)(C3)O1)C2 |r,TLB:23:24:21.22.31:32,27:26:21:33.23.24,THB:23:22:32:33.24.25,25:24:21:31.26.32,25:26:21:33.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557515
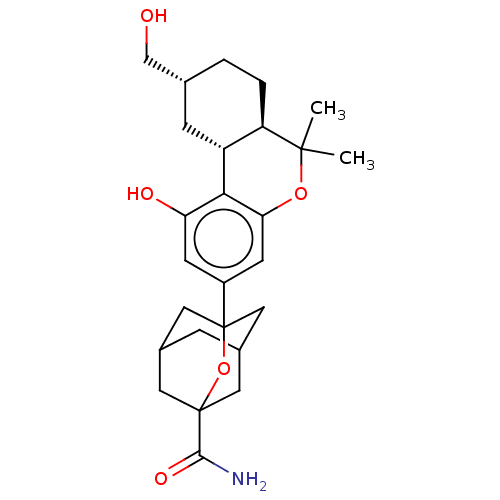
(CHEMBL4744219)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:24:25:22.23.28:29,30:27:22:33.24.25,THB:24:23:29:33.25.26,26:25:22:28.27.29,26:27:22:33.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50099679
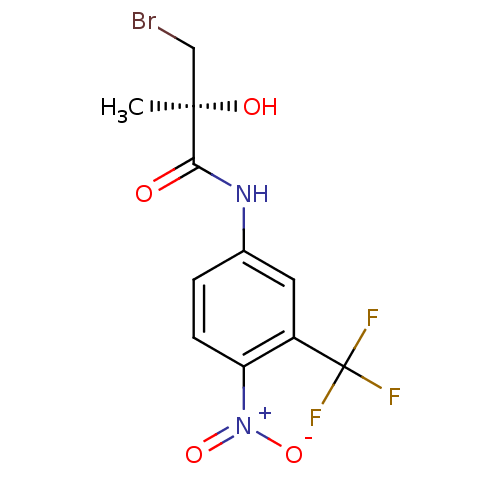
(3-Bromo-2-hydroxy-2-methyl-N-(4-nitro-3-trifluorom...)Show SMILES C[C@@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC9 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557512

(CHEMBL4779775)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C#N)C2 |r,TLB:23:24:21.22.27:28,29:26:21:31.23.24,THB:23:22:28:31.24.25,25:24:21:27.26.28,25:26:21:31.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50557511
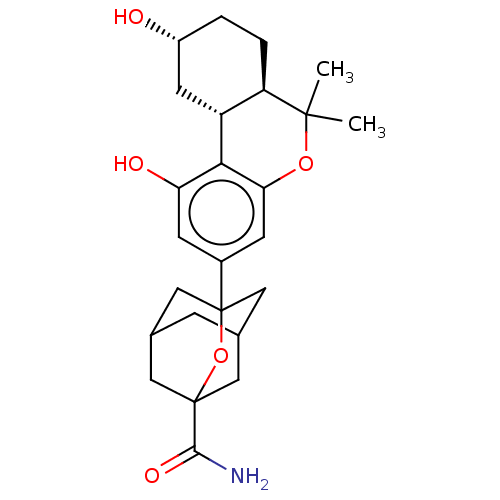
(CHEMBL4764017)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:23:24:21.22.27:28,29:26:21:32.23.24,THB:23:22:28:32.24.25,25:24:21:27.26.28,25:26:21:32.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615069
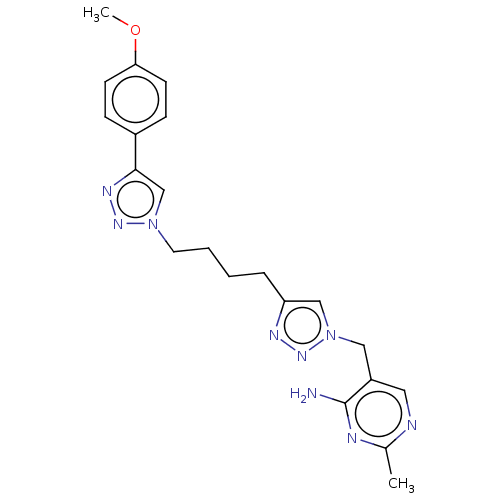
(CHEMBL5271689)Show SMILES COc1ccc(cc1)-c1cn(CCCCc2cn(Cc3cnc(C)nc3N)nn2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557512

(CHEMBL4779775)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C#N)C2 |r,TLB:23:24:21.22.27:28,29:26:21:31.23.24,THB:23:22:28:31.24.25,25:24:21:27.26.28,25:26:21:31.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50557515

(CHEMBL4744219)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:24:25:22.23.28:29,30:27:22:33.24.25,THB:24:23:29:33.25.26,26:25:22:28.27.29,26:27:22:33.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC7 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain CB1 receptor by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615071

(CHEMBL5290932) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615070

(CHEMBL5268763) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC5 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50099680
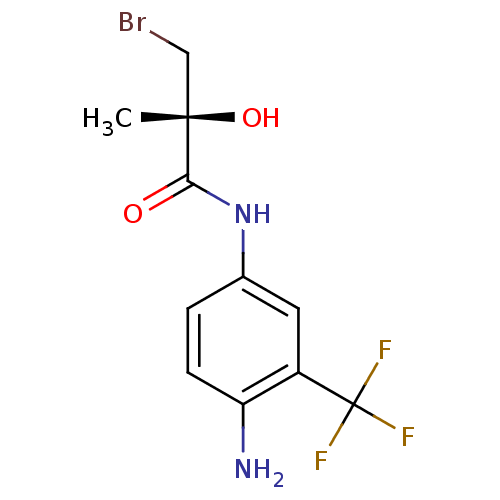
((R)-N-(4-Amino-3-trifluoromethyl-phenyl)-3-bromo-2...)Show InChI InChI=1S/C11H12BrF3N2O2/c1-10(19,5-12)9(18)17-6-2-3-8(16)7(4-6)11(13,14)15/h2-4,19H,5,16H2,1H3,(H,17,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557515

(CHEMBL4744219)Show SMILES [H][C@@]12C[C@H](CO)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:24:25:22.23.28:29,30:27:22:33.24.25,THB:24:23:29:33.25.26,26:25:22:28.27.29,26:27:22:33.24.25| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC4 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM207628
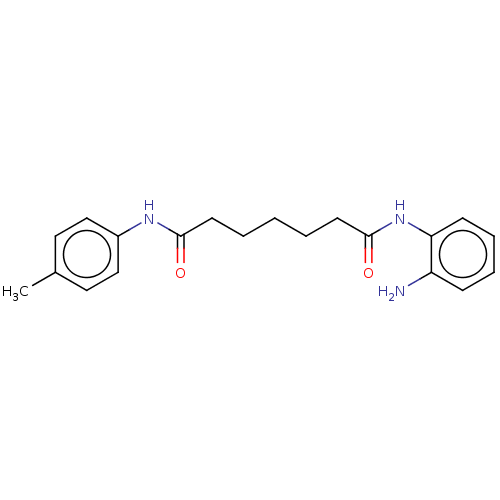
(US9265734, RGFA8 | US9796664, Compound RGFA8)Show InChI InChI=1S/C20H25N3O2/c1-15-11-13-16(14-12-15)22-19(24)9-3-2-4-10-20(25)23-18-8-6-5-7-17(18)21/h5-8,11-14H,2-4,9-10,21H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG/His-tagged HDAC1 (1 to 482 residues) expressed in sf9 cells using acetyl-Lys(Ac)-AMC as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50557511

(CHEMBL4764017)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:23:24:21.22.27:28,29:26:21:32.23.24,THB:23:22:28:32.24.25,25:24:21:27.26.28,25:26:21:32.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50565137
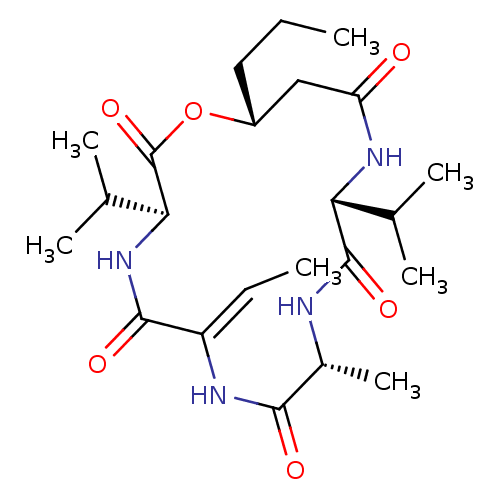
(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC2 expressed in Sf9 cells using Ac-Leu-Gly-Lys(Ac)-AMC as substrate measured every 30 secs for 60 mins by fluorescence-based a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50565137

(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using Ac-Leu-Gly-Lys(Ac)-AMC as substrate measured every 30 secs for 60 mins by fluorescence-based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50565137

(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human C-terminal His-tagged HDAC3 (1 to 428 residues)/human N-terminal GST-tagged NcoR2 (395 to 489 residues) expressed in sf9 cells us... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50557511

(CHEMBL4764017)Show SMILES [H][C@@]12C[C@H](O)CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C12CC3CC(CC(C3)(O1)C(N)=O)C2 |r,TLB:23:24:21.22.27:28,29:26:21:32.23.24,THB:23:22:28:32.24.25,25:24:21:27.26.28,25:26:21:32.23.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membrane by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127882
BindingDB Entry DOI: 10.7270/Q2NP283T |
More data for this
Ligand-Target Pair | |
Kallikrein-2
(Homo sapiens (Human)) | BDBM50292533
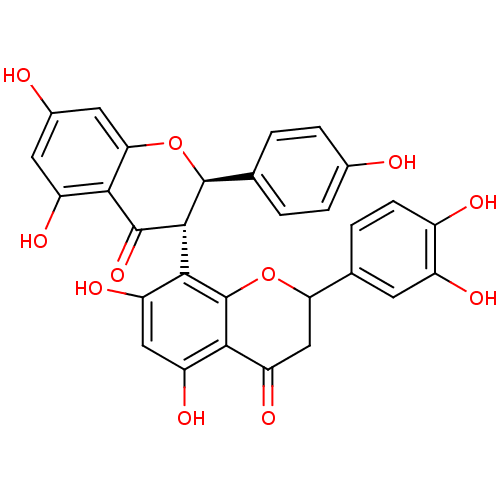
(CHEMBL446790 | Fukugetin)Show SMILES Oc1ccc(cc1)[C@@H]1Oc2cc(O)cc(O)c2C(=O)[C@H]1c1c(O)cc(O)c2C(=O)CC(Oc12)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C30H22O11/c31-14-4-1-12(2-5-14)29-27(28(39)25-18(35)8-15(32)9-23(25)41-29)26-20(37)10-19(36)24-21(38)11-22(40-30(24)26)13-3-6-16(33)17(34)7-13/h1-10,22,27,29,31-37H,11H2/t22?,27-,29+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Campus Inc
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human tissue kallikrein-2 expressed in baculovirus infected insect cells using Abz-KLRSSQ-EDDnp as substrate preincubated fo... |
Bioorg Med Chem Lett 26: 1485-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.039
BindingDB Entry DOI: 10.7270/Q2862J9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 residues 379-382 (RHKKAc) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data