 Found 125 hits with Last Name = 'choon' and Initial = 'ts'
Found 125 hits with Last Name = 'choon' and Initial = 'ts' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360916
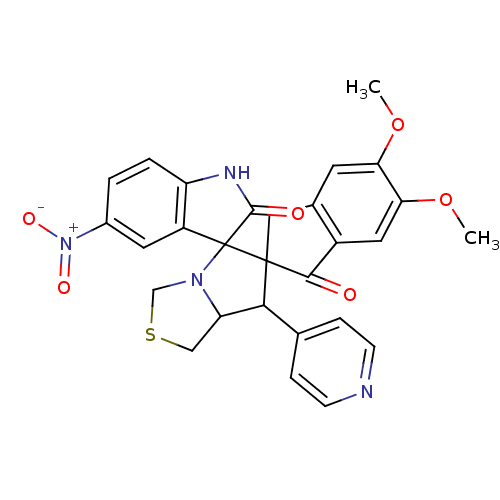
(CHEMBL1935151)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccncc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H24N4O6S/c1-37-22-9-16-12-27(25(33)18(16)11-23(22)38-2)24(15-5-7-29-8-6-15)21-13-39-14-31(21)28(27)19-10-17(32(35)36)3-4-20(19)30-26(28)34/h3-11,21,24H,12-14H2,1-2H3,(H,30,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Bioorg Med Chem Lett 23: 2101-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.122
BindingDB Entry DOI: 10.7270/Q2BZ67DX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360911
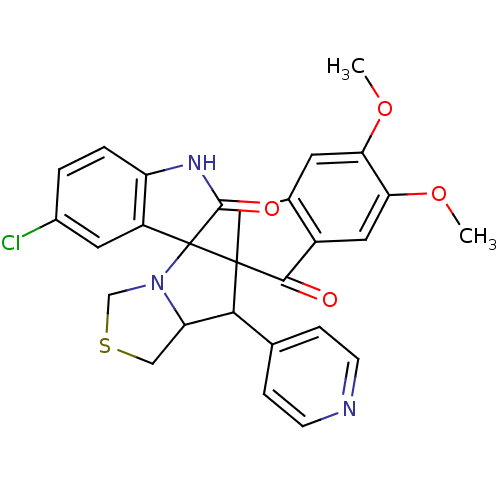
(CHEMBL1935146)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(Cl)cc34)c3ccncc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H24ClN3O4S/c1-35-22-9-16-12-27(25(33)18(16)11-23(22)36-2)24(15-5-7-30-8-6-15)21-13-37-14-32(21)28(27)19-10-17(29)3-4-20(19)31-26(28)34/h3-11,21,24H,12-14H2,1-2H3,(H,31,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360906
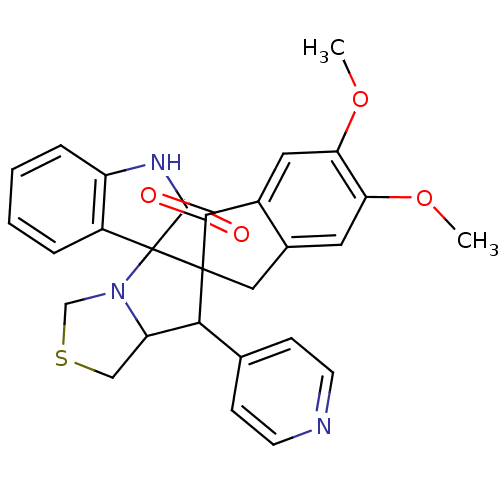
(CHEMBL1935141)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccccc34)c3ccncc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H25N3O4S/c1-34-22-11-17-13-27(25(32)18(17)12-23(22)35-2)24(16-7-9-29-10-8-16)21-14-36-15-31(21)28(27)19-5-3-4-6-20(19)30-26(28)33/h3-12,21,24H,13-15H2,1-2H3,(H,30,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50346981
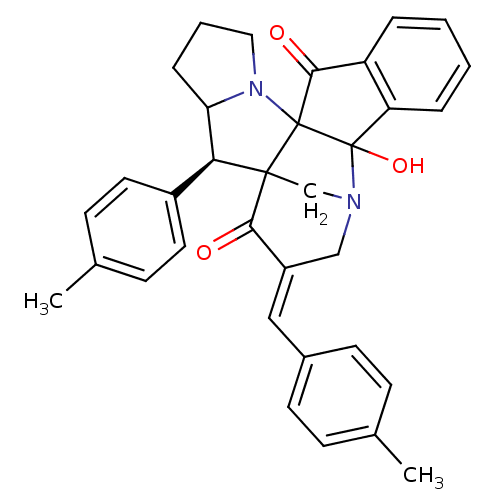
(CHEMBL1795895)Show SMILES Cc1ccc(\C=C2/CN3CC4([C@H](C5CCCN5C44C(=O)c5ccccc5C34O)c3ccc(C)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C34H32N2O3/c1-21-9-13-23(14-10-21)18-25-19-35-20-32(30(25)37)29(24-15-11-22(2)12-16-24)28-8-5-17-36(28)33(32)31(38)26-6-3-4-7-27(26)34(33,35)39/h3-4,6-7,9-16,18,28-29,39H,5,8,17,19-20H2,1-2H3/b25-18+/t28?,29-,32?,33?,34?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360907
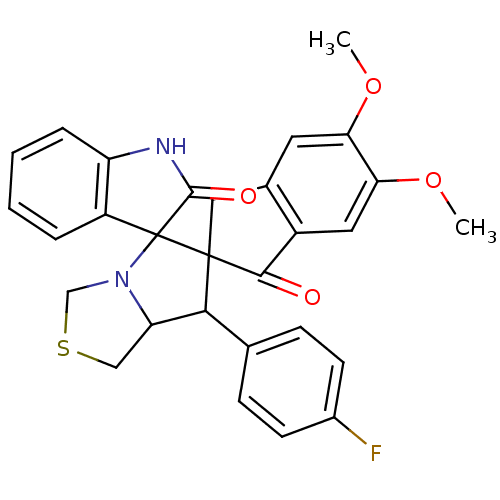
(CHEMBL1935142)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccccc34)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H25FN2O4S/c1-35-23-11-17-13-28(26(33)19(17)12-24(23)36-2)25(16-7-9-18(30)10-8-16)22-14-37-15-32(22)29(28)20-5-3-4-6-21(20)31-27(29)34/h3-12,22,25H,13-15H2,1-2H3,(H,31,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360917
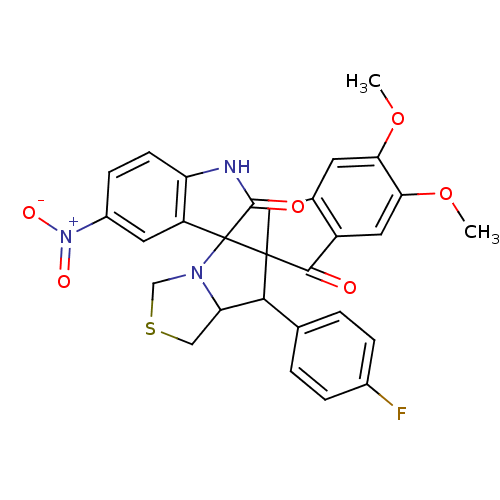
(CHEMBL1935152)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H24FN3O6S/c1-38-23-9-16-12-28(26(34)19(16)11-24(23)39-2)25(15-3-5-17(30)6-4-15)22-13-40-14-32(22)29(28)20-10-18(33(36)37)7-8-21(20)31-27(29)35/h3-11,22,25H,12-14H2,1-2H3,(H,31,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50346977
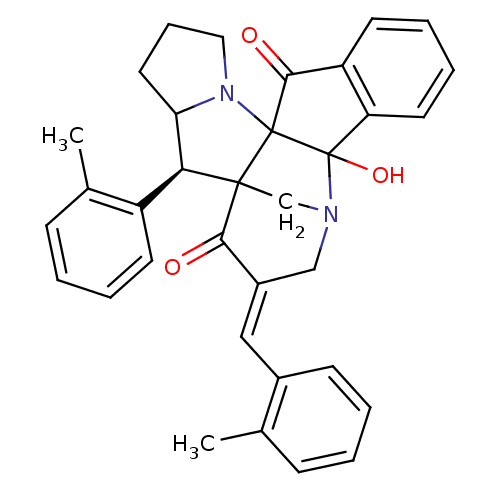
(CHEMBL1795891)Show SMILES Cc1ccccc1\C=C1/CN2CC3([C@H](C4CCCN4C33C(=O)c4ccccc4C23O)c2ccccc2C)C1=O |r| Show InChI InChI=1S/C34H32N2O3/c1-21-10-3-5-12-23(21)18-24-19-35-20-32(30(24)37)29(25-13-6-4-11-22(25)2)28-16-9-17-36(28)33(32)31(38)26-14-7-8-15-27(26)34(33,35)39/h3-8,10-15,18,28-29,39H,9,16-17,19-20H2,1-2H3/b24-18+/t28?,29-,32?,33?,34?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360912
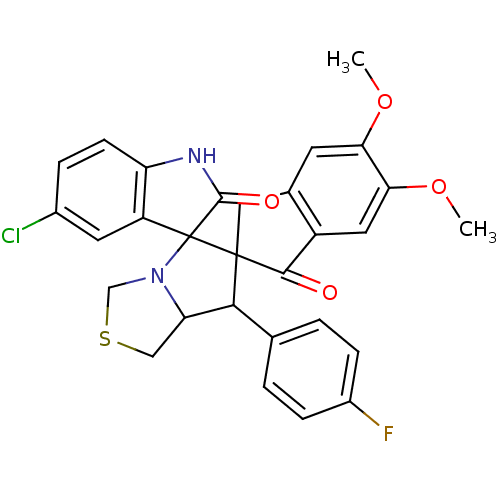
(CHEMBL1935147)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(Cl)cc34)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H24ClFN2O4S/c1-36-23-9-16-12-28(26(34)19(16)11-24(23)37-2)25(15-3-6-18(31)7-4-15)22-13-38-14-33(22)29(28)20-10-17(30)5-8-21(20)32-27(29)35/h3-11,22,25H,12-14H2,1-2H3,(H,32,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436816
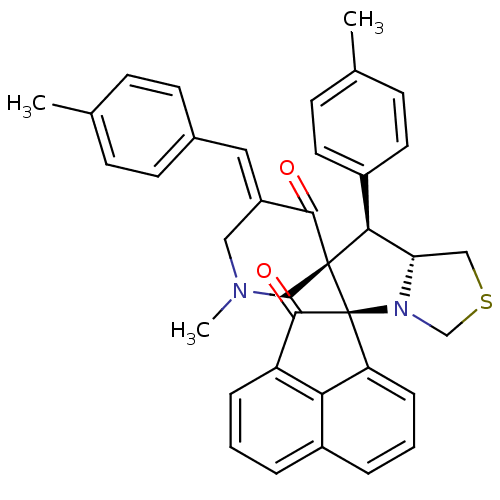
(CHEMBL3038175)Show SMILES CN1C\C(=C/c2ccc(C)cc2)C(=O)[C@]2(C1)[C@H]([C@@H]1CSCN1[C@@]21C(=O)c2cccc3cccc1c23)c1ccc(C)cc1 |r| Show InChI InChI=1S/C37H34N2O2S/c1-23-10-14-25(15-11-23)18-28-19-38(3)21-36(34(28)40)33(27-16-12-24(2)13-17-27)31-20-42-22-39(31)37(36)30-9-5-7-26-6-4-8-29(32(26)30)35(37)41/h4-18,31,33H,19-22H2,1-3H3/b28-18+/t31-,33-,36-,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360918
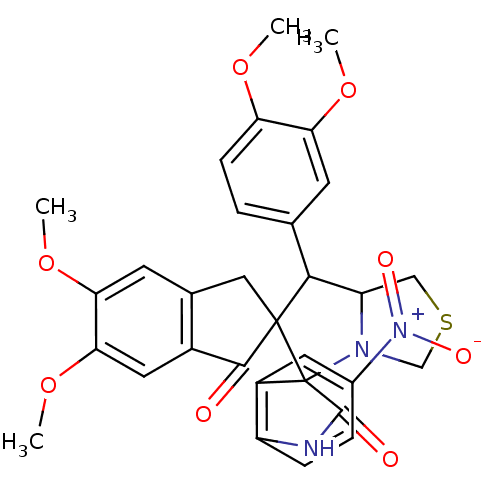
(CHEMBL1935153)Show SMILES COc1ccc(cc1OC)C1C2CSCN2C2(C(=O)Nc3ccc(cc23)[N+]([O-])=O)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C31H29N3O8S/c1-39-23-8-5-16(9-24(23)40-2)27-22-14-43-15-33(22)31(20-11-18(34(37)38)6-7-21(20)32-29(31)36)30(27)13-17-10-25(41-3)26(42-4)12-19(17)28(30)35/h5-12,22,27H,13-15H2,1-4H3,(H,32,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378858
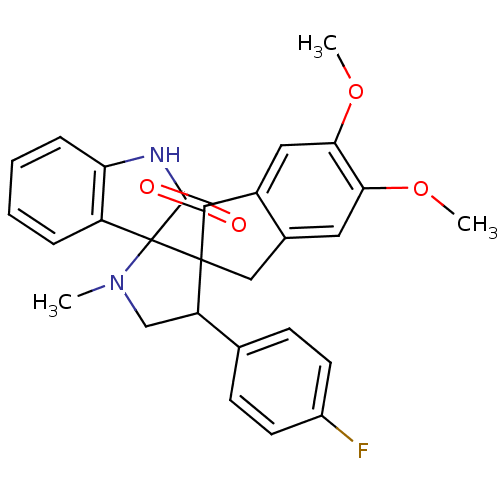
(CHEMBL1289045)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccccc34)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H25FN2O4/c1-31-15-21(16-8-10-18(29)11-9-16)27(28(31)20-6-4-5-7-22(20)30-26(28)33)14-17-12-23(34-2)24(35-3)13-19(17)25(27)32/h4-13,21H,14-15H2,1-3H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50346985
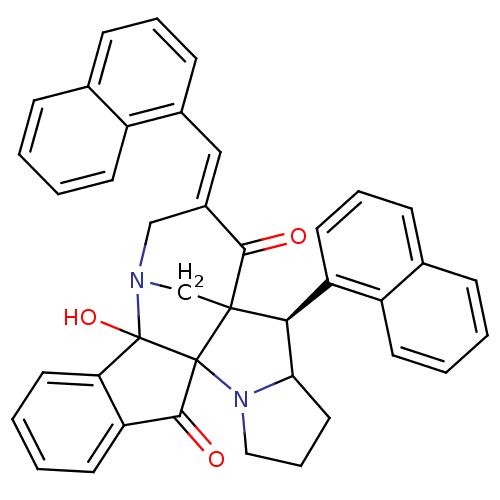
(CHEMBL1795899)Show SMILES OC12N3CC4([C@H](C5CCCN5C14C(=O)c1ccccc21)c1cccc2ccccc12)C(=O)\C(C3)=C\c1cccc2ccccc12 |r| Show InChI InChI=1S/C40H32N2O3/c43-36-28(22-27-14-7-12-25-10-1-3-15-29(25)27)23-41-24-38(36)35(31-18-8-13-26-11-2-4-16-30(26)31)34-20-9-21-42(34)39(38)37(44)32-17-5-6-19-33(32)40(39,41)45/h1-8,10-19,22,34-35,45H,9,20-21,23-24H2/b28-22+/t34?,35-,38?,39?,40?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360919
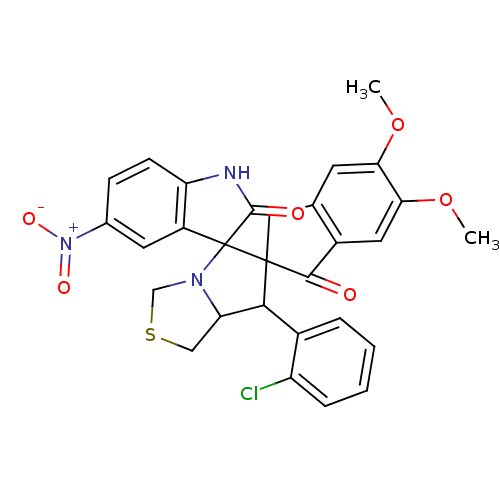
(CHEMBL1935154)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C29H24ClN3O6S/c1-38-23-9-15-12-28(26(34)18(15)11-24(23)39-2)25(17-5-3-4-6-20(17)30)22-13-40-14-32(22)29(28)19-10-16(33(36)37)7-8-21(19)31-27(29)35/h3-11,22,25H,12-14H2,1-2H3,(H,31,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360913
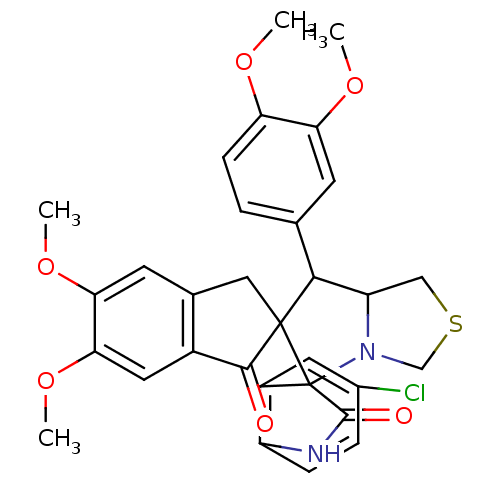
(CHEMBL1935148)Show SMILES COc1ccc(cc1OC)C1C2CSCN2C2(C(=O)Nc3ccc(Cl)cc23)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C31H29ClN2O6S/c1-37-23-8-5-16(9-24(23)38-2)27-22-14-41-15-34(22)31(20-11-18(32)6-7-21(20)33-29(31)36)30(27)13-17-10-25(39-3)26(40-4)12-19(17)28(30)35/h5-12,22,27H,13-15H2,1-4H3,(H,33,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436833
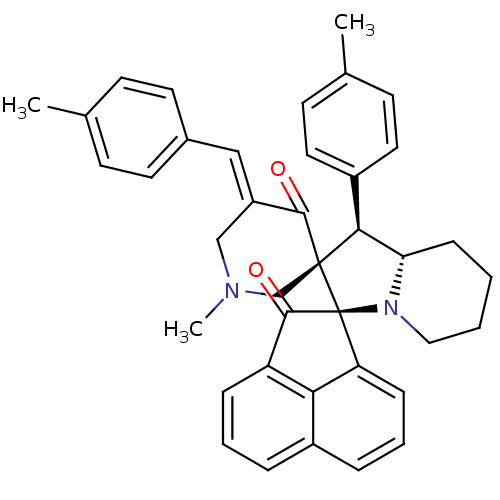
(CHEMBL3038155)Show SMILES CN1C\C(=C/c2ccc(C)cc2)C(=O)[C@]2(C1)[C@H]([C@@H]1CCCCN1[C@@]21C(=O)c2cccc3cccc1c23)c1ccc(C)cc1 |r| Show InChI InChI=1S/C39H38N2O2/c1-25-13-17-27(18-14-25)22-30-23-40(3)24-38(36(30)42)35(29-19-15-26(2)16-20-29)33-12-4-5-21-41(33)39(38)32-11-7-9-28-8-6-10-31(34(28)32)37(39)43/h6-11,13-20,22,33,35H,4-5,12,21,23-24H2,1-3H3/b30-22+/t33-,35-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360915
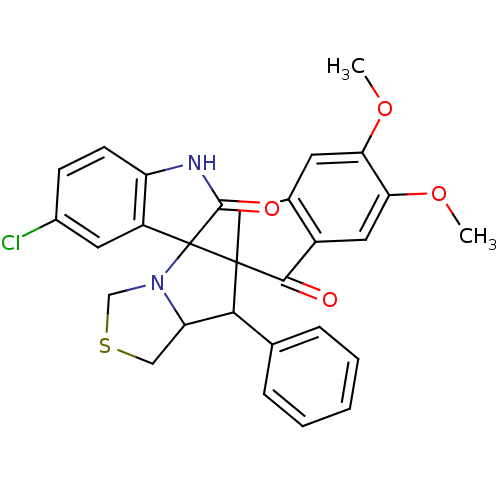
(CHEMBL1935150)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(Cl)cc34)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H25ClN2O4S/c1-35-23-10-17-13-28(26(33)19(17)12-24(23)36-2)25(16-6-4-3-5-7-16)22-14-37-15-32(22)29(28)20-11-18(30)8-9-21(20)31-27(29)34/h3-12,22,25H,13-15H2,1-2H3,(H,31,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378860
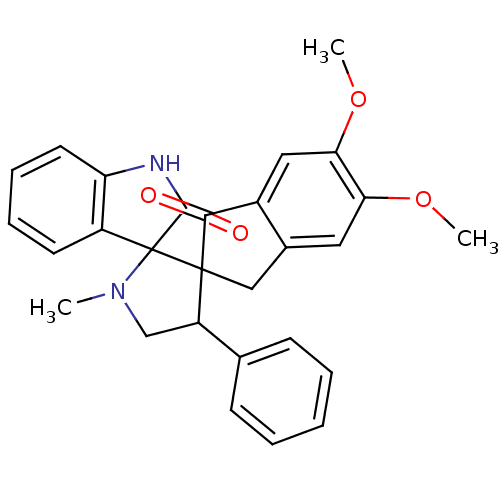
(CHEMBL1289160)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccccc34)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H26N2O4/c1-30-16-21(17-9-5-4-6-10-17)27(28(30)20-11-7-8-12-22(20)29-26(28)32)15-18-13-23(33-2)24(34-3)14-19(18)25(27)31/h4-14,21H,15-16H2,1-3H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360920
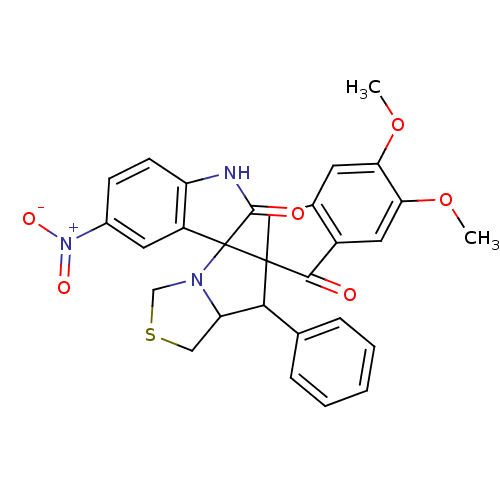
(CHEMBL1935155)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H25N3O6S/c1-37-23-10-17-13-28(26(33)19(17)12-24(23)38-2)25(16-6-4-3-5-7-16)22-14-39-15-31(22)29(28)20-11-18(32(35)36)8-9-21(20)30-27(29)34/h3-12,22,25H,13-15H2,1-2H3,(H,30,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360914
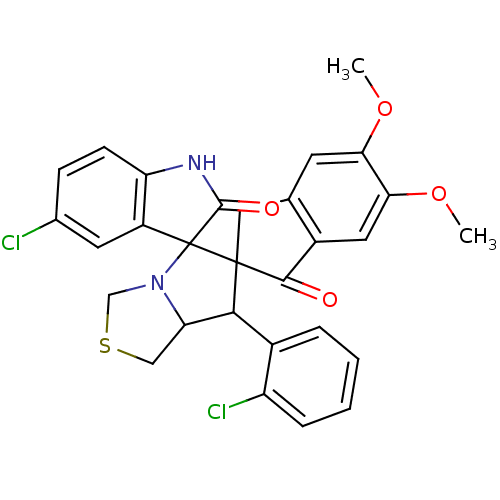
(CHEMBL1935149)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccc(Cl)cc34)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C29H24Cl2N2O4S/c1-36-23-9-15-12-28(26(34)18(15)11-24(23)37-2)25(17-5-3-4-6-20(17)31)22-13-38-14-33(22)29(28)19-10-16(30)7-8-21(19)32-27(29)35/h3-11,22,25H,12-14H2,1-2H3,(H,32,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436819

(CHEMBL3038151)Show SMILES CN1C\C(=C/c2cccc3ccccc23)C(=O)[C@]2(C1)[C@H]([C@@H]1CCCCN1[C@@]21C(=O)c2cccc3cccc1c23)c1cccc2ccccc12 |r| Show InChI InChI=1S/C45H38N2O2/c1-46-27-33(26-32-18-8-14-29-12-2-4-19-34(29)32)42(48)44(28-46)41(36-21-9-15-30-13-3-5-20-35(30)36)39-24-6-7-25-47(39)45(44)38-23-11-17-31-16-10-22-37(40(31)38)43(45)49/h2-5,8-23,26,39,41H,6-7,24-25,27-28H2,1H3/b33-26+/t39-,41-,44-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378880
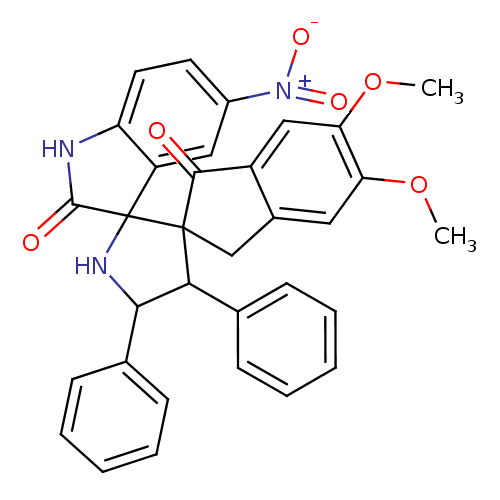
(CHEMBL1288828)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccccc3)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C33H27N3O6/c1-41-26-15-21-18-32(30(37)23(21)17-27(26)42-2)28(19-9-5-3-6-10-19)29(20-11-7-4-8-12-20)35-33(32)24-16-22(36(39)40)13-14-25(24)34-31(33)38/h3-17,28-29,35H,18H2,1-2H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378862
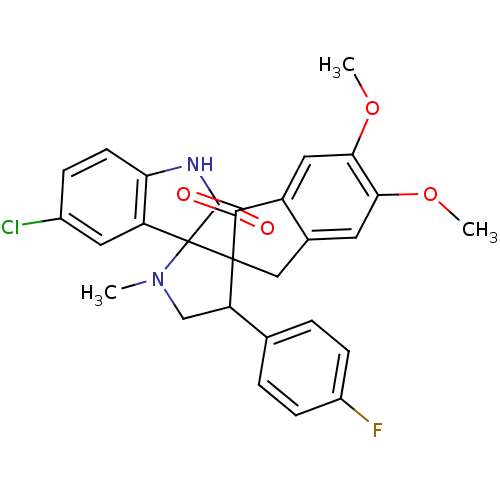
(CHEMBL1289274)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccc(Cl)cc34)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C28H24ClFN2O4/c1-32-14-21(15-4-7-18(30)8-5-15)27(28(32)20-11-17(29)6-9-22(20)31-26(28)34)13-16-10-23(35-2)24(36-3)12-19(16)25(27)33/h4-12,21H,13-14H2,1-3H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436827
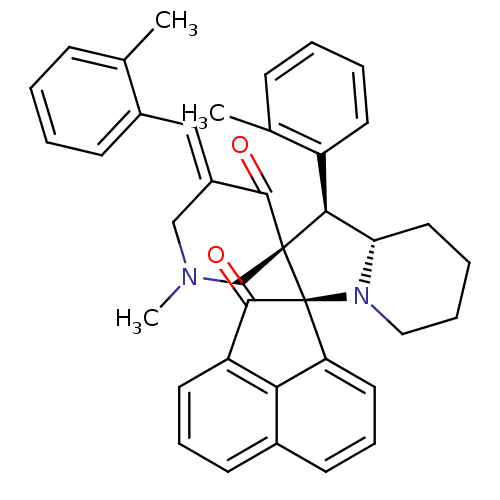
(CHEMBL3038161)Show SMILES CN1C\C(=C/c2ccccc2C)C(=O)[C@]2(C1)[C@H]([C@@H]1CCCCN1[C@@]21C(=O)c2cccc3cccc1c23)c1ccccc1C |r| Show InChI InChI=1S/C39H38N2O2/c1-25-12-4-6-14-28(25)22-29-23-40(3)24-38(36(29)42)35(30-17-7-5-13-26(30)2)33-20-8-9-21-41(33)39(38)32-19-11-16-27-15-10-18-31(34(27)32)37(39)43/h4-7,10-19,22,33,35H,8-9,20-21,23-24H2,1-3H3/b29-22+/t33-,35-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50431817
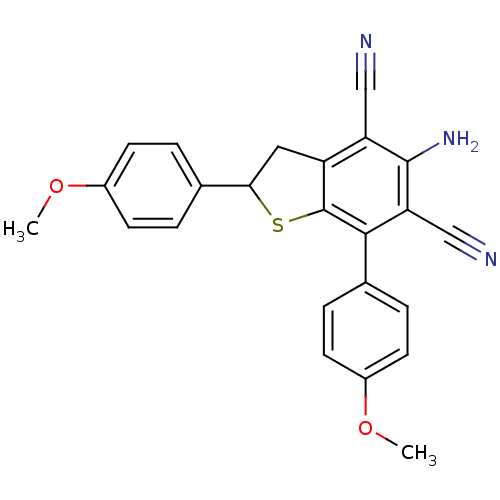
(CHEMBL2347105)Show SMILES COc1ccc(cc1)C1Cc2c(S1)c(-c1ccc(OC)cc1)c(C#N)c(N)c2C#N Show InChI InChI=1S/C24H19N3O2S/c1-28-16-7-3-14(4-8-16)21-11-18-19(12-25)23(27)20(13-26)22(24(18)30-21)15-5-9-17(29-2)10-6-15/h3-10,21H,11,27H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Bioorg Med Chem Lett 23: 2101-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.122
BindingDB Entry DOI: 10.7270/Q2BZ67DX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378859
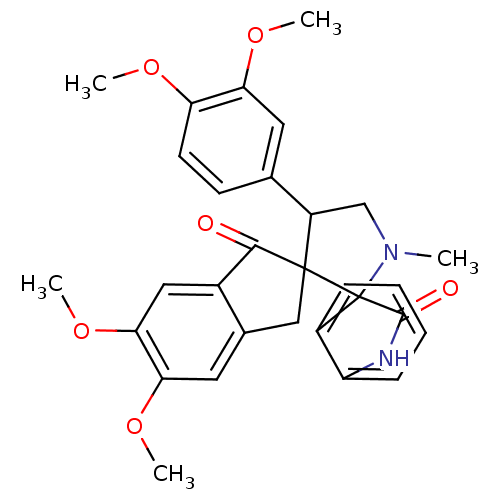
(CHEMBL1289159)Show SMILES COc1ccc(cc1OC)C1CN(C)C2(C(=O)Nc3ccccc23)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C30H30N2O6/c1-32-16-21(17-10-11-23(35-2)24(12-17)36-3)29(30(32)20-8-6-7-9-22(20)31-28(30)34)15-18-13-25(37-4)26(38-5)14-19(18)27(29)33/h6-14,21H,15-16H2,1-5H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378881
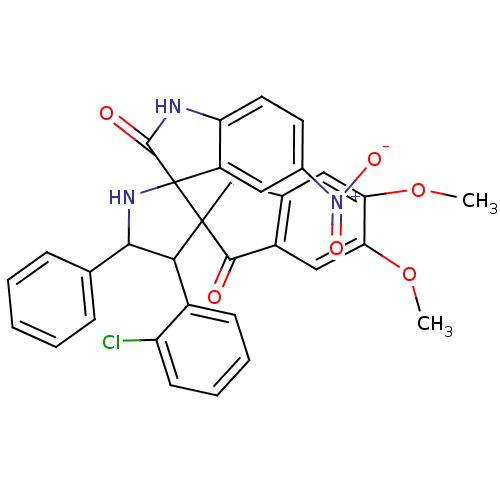
(CHEMBL1288829)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccccc3)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C33H26ClN3O6/c1-42-26-14-19-17-32(30(38)22(19)16-27(26)43-2)28(21-10-6-7-11-24(21)34)29(18-8-4-3-5-9-18)36-33(32)23-15-20(37(40)41)12-13-25(23)35-31(33)39/h3-16,28-29,36H,17H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378863
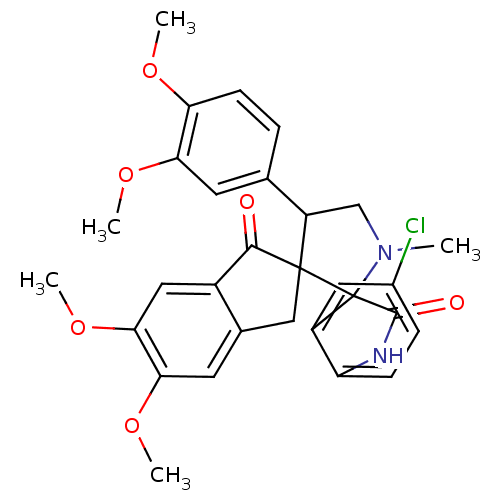
(CHEMBL1289391)Show SMILES COc1ccc(cc1OC)C1CN(C)C2(C(=O)Nc3ccc(Cl)cc23)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C30H29ClN2O6/c1-33-15-21(16-6-9-23(36-2)24(10-16)37-3)29(30(33)20-12-18(31)7-8-22(20)32-28(30)35)14-17-11-25(38-4)26(39-5)13-19(17)27(29)34/h6-13,21H,14-15H2,1-5H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50431830
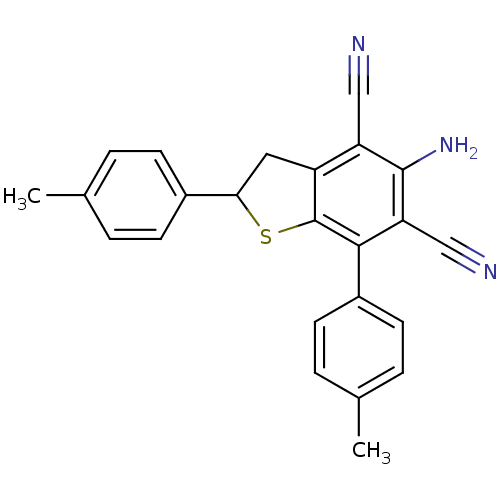
(CHEMBL2347092)Show SMILES Cc1ccc(cc1)C1Cc2c(S1)c(-c1ccc(C)cc1)c(C#N)c(N)c2C#N Show InChI InChI=1S/C24H19N3S/c1-14-3-7-16(8-4-14)21-11-18-19(12-25)23(27)20(13-26)22(24(18)28-21)17-9-5-15(2)6-10-17/h3-10,21H,11,27H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Bioorg Med Chem Lett 23: 2101-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.122
BindingDB Entry DOI: 10.7270/Q2BZ67DX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378861
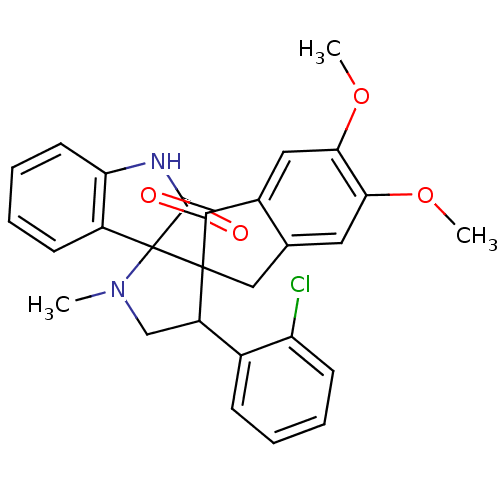
(CHEMBL1289273)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccccc34)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C28H25ClN2O4/c1-31-15-20(17-8-4-6-10-21(17)29)27(28(31)19-9-5-7-11-22(19)30-26(28)33)14-16-12-23(34-2)24(35-3)13-18(16)25(27)32/h4-13,20H,14-15H2,1-3H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436812
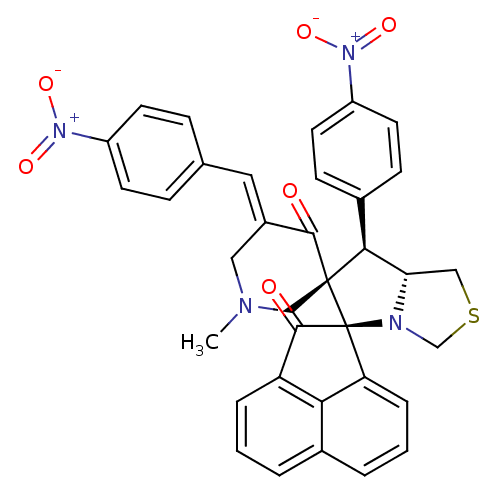
(CHEMBL3038164)Show SMILES CN1C\C(=C/c2ccc(cc2)[N+]([O-])=O)C(=O)[C@]2(C1)[C@H]([C@@H]1CSCN1[C@@]21C(=O)c2cccc3cccc1c23)c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C35H28N4O6S/c1-36-17-24(16-21-8-12-25(13-9-21)38(42)43)32(40)34(19-36)31(23-10-14-26(15-11-23)39(44)45)29-18-46-20-37(29)35(34)28-7-3-5-22-4-2-6-27(30(22)28)33(35)41/h2-16,29,31H,17-20H2,1H3/b24-16+/t29-,31-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
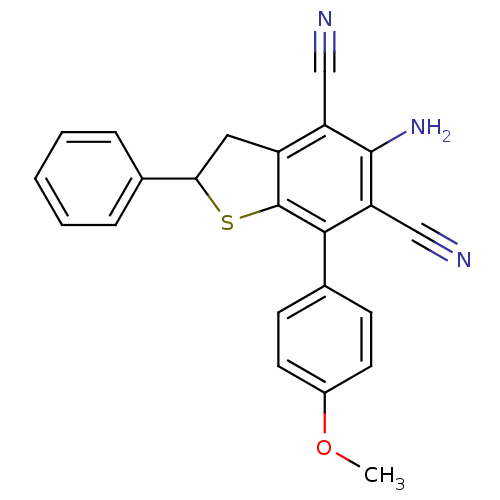
(Electrophorus electricus (Electric eel)) | BDBM50431835
(CHEMBL2347087)Show SMILES COc1ccc(cc1)-c1c2SC(Cc2c(C#N)c(N)c1C#N)c1ccccc1 Show InChI InChI=1S/C23H17N3OS/c1-27-16-9-7-15(8-10-16)21-19(13-25)22(26)18(12-24)17-11-20(28-23(17)21)14-5-3-2-4-6-14/h2-10,20H,11,26H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Bioorg Med Chem Lett 23: 2101-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.122
BindingDB Entry DOI: 10.7270/Q2BZ67DX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50346978
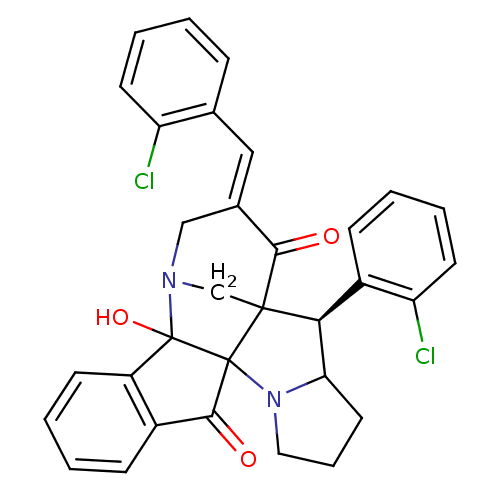
(CHEMBL1795892)Show SMILES OC12N3CC4([C@H](C5CCCN5C14C(=O)c1ccccc21)c1ccccc1Cl)C(=O)\C(C3)=C\c1ccccc1Cl |r| Show InChI InChI=1S/C32H26Cl2N2O3/c33-24-12-5-1-8-19(24)16-20-17-35-18-30(28(20)37)27(22-10-3-6-13-25(22)34)26-14-7-15-36(26)31(30)29(38)21-9-2-4-11-23(21)32(31,35)39/h1-6,8-13,16,26-27,39H,7,14-15,17-18H2/b20-16+/t26?,27-,30?,31?,32?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378872
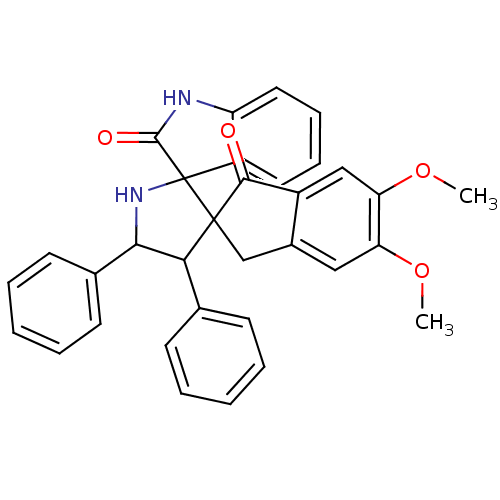
(CHEMBL1289942)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccccc34)c3ccccc3)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C33H28N2O4/c1-38-26-17-22-19-32(30(36)23(22)18-27(26)39-2)28(20-11-5-3-6-12-20)29(21-13-7-4-8-14-21)35-33(32)24-15-9-10-16-25(24)34-31(33)37/h3-18,28-29,35H,19H2,1-2H3,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378871
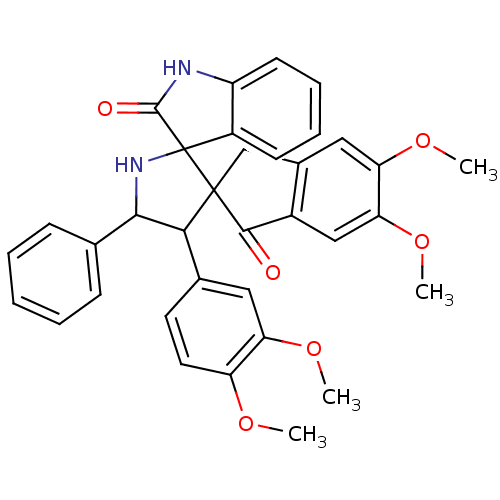
(CHEMBL1288822)Show SMILES COc1ccc(cc1OC)C1C(NC2(C(=O)Nc3ccccc23)C11Cc2cc(OC)c(OC)cc2C1=O)c1ccccc1 Show InChI InChI=1S/C35H32N2O6/c1-40-26-15-14-21(16-27(26)41-2)30-31(20-10-6-5-7-11-20)37-35(24-12-8-9-13-25(24)36-33(35)39)34(30)19-22-17-28(42-3)29(43-4)18-23(22)32(34)38/h5-18,30-31,37H,19H2,1-4H3,(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
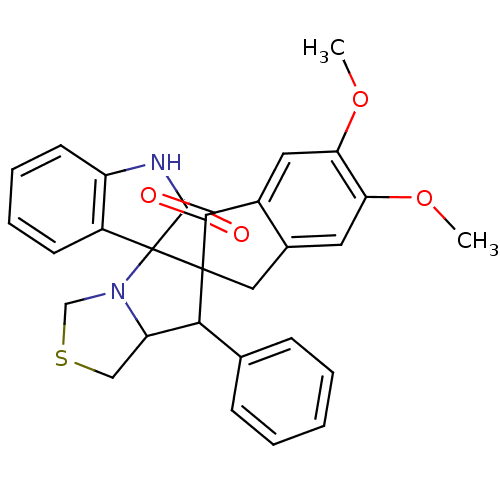
(Electrophorus electricus (Electric eel)) | BDBM50360910
(CHEMBL1935145)Show SMILES COc1cc2CC3(C(C4CSCN4C33C(=O)Nc4ccccc34)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C29H26N2O4S/c1-34-23-12-18-14-28(26(32)19(18)13-24(23)35-2)25(17-8-4-3-5-9-17)22-15-36-16-31(22)29(28)20-10-6-7-11-21(20)30-27(29)33/h3-13,22,25H,14-16H2,1-2H3,(H,30,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
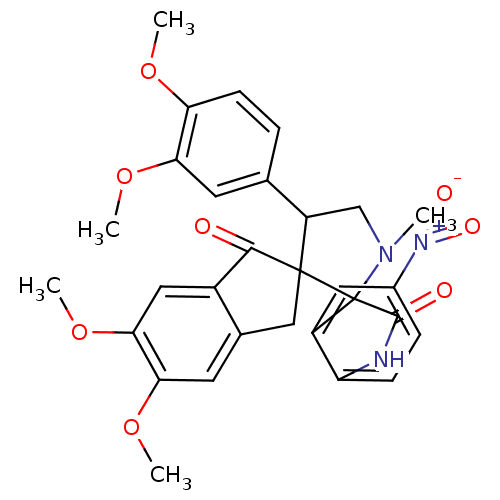
(Homo sapiens (Human)) | BDBM50378867
(CHEMBL1288821)Show SMILES COc1ccc(cc1OC)C1CN(C)C2(C(=O)Nc3ccc(cc23)[N+]([O-])=O)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C30H29N3O8/c1-32-15-21(16-6-9-23(38-2)24(10-16)39-3)29(14-17-11-25(40-4)26(41-5)13-19(17)27(29)34)30(32)20-12-18(33(36)37)7-8-22(20)31-28(30)35/h6-13,21H,14-15H2,1-5H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
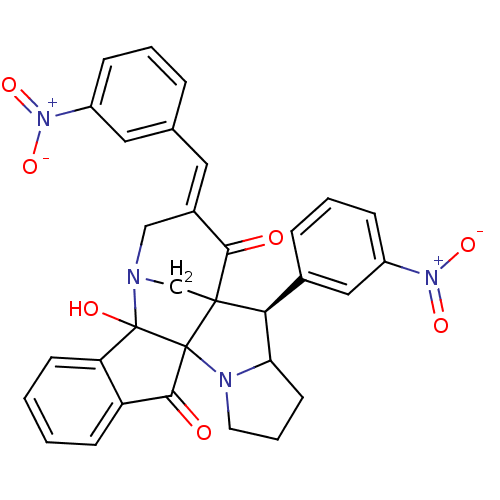
(Electrophorus electricus (Electric eel)) | BDBM50346980
(CHEMBL1795894)Show SMILES OC12N3CC4([C@H](C5CCCN5C14C(=O)c1ccccc21)c1cccc(c1)[N+]([O-])=O)C(=O)\C(C3)=C\c1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C32H26N4O7/c37-28-21(14-19-6-3-8-22(15-19)35(40)41)17-33-18-30(28)27(20-7-4-9-23(16-20)36(42)43)26-12-5-13-34(26)31(30)29(38)24-10-1-2-11-25(24)32(31,33)39/h1-4,6-11,14-16,26-27,39H,5,12-13,17-18H2/b21-14+/t26?,27-,30?,31?,32?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50360908
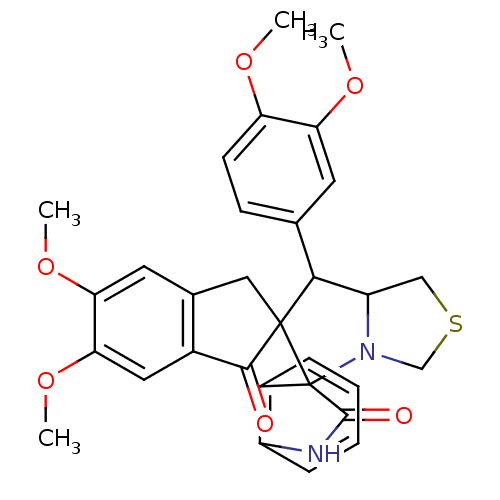
(CHEMBL1935143)Show SMILES COc1ccc(cc1OC)C1C2CSCN2C2(C(=O)Nc3ccccc23)C11Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C31H30N2O6S/c1-36-23-10-9-17(11-24(23)37-2)27-22-15-40-16-33(22)31(20-7-5-6-8-21(20)32-29(31)35)30(27)14-18-12-25(38-3)26(39-4)13-19(18)28(30)34/h5-13,22,27H,14-16H2,1-4H3,(H,32,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine as substrate measured every sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 508-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.087
BindingDB Entry DOI: 10.7270/Q21Z44WD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50436810
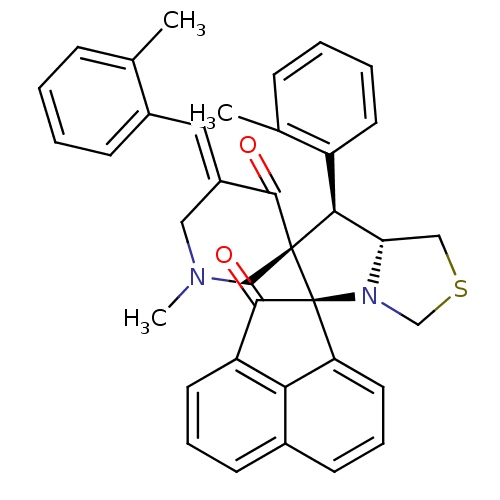
(CHEMBL3038166)Show SMILES CN1C\C(=C/c2ccccc2C)C(=O)[C@]2(C1)[C@H]([C@@H]1CSCN1[C@@]21C(=O)c2cccc3cccc1c23)c1ccccc1C |r| Show InChI InChI=1S/C37H34N2O2S/c1-23-10-4-6-12-26(23)18-27-19-38(3)21-36(34(27)40)33(28-15-7-5-11-24(28)2)31-20-42-22-39(31)37(36)30-17-9-14-25-13-8-16-29(32(25)30)35(37)41/h4-18,31,33H,19-22H2,1-3H3/b27-18+/t31-,33-,36-,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE after 30 mins by Ellman's method |
Eur J Med Chem 65: 240-8 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.050
BindingDB Entry DOI: 10.7270/Q23T9JNC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378865
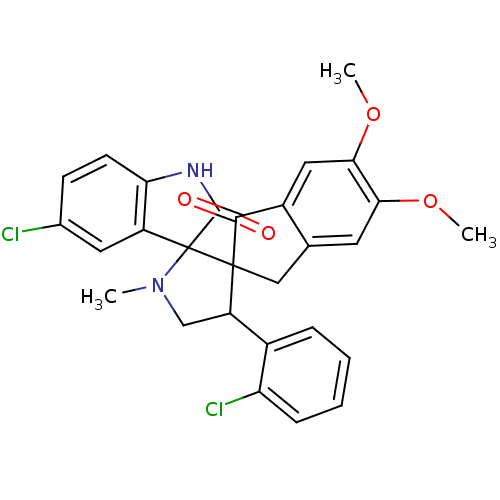
(CHEMBL1289506)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccc(Cl)cc34)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C28H24Cl2N2O4/c1-32-14-20(17-6-4-5-7-21(17)30)27(28(32)19-11-16(29)8-9-22(19)31-26(28)34)13-15-10-23(35-2)24(36-3)12-18(15)25(27)33/h4-12,20H,13-14H2,1-3H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378870
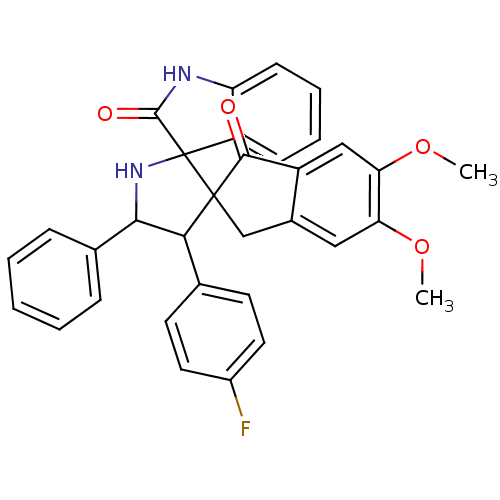
(CHEMBL1289825)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccccc34)c3ccccc3)c3ccc(F)cc3)C(=O)c2cc1OC Show InChI InChI=1S/C33H27FN2O4/c1-39-26-16-21-18-32(30(37)23(21)17-27(26)40-2)28(19-12-14-22(34)15-13-19)29(20-8-4-3-5-9-20)36-33(32)24-10-6-7-11-25(24)35-31(33)38/h3-17,28-29,36H,18H2,1-2H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50346976
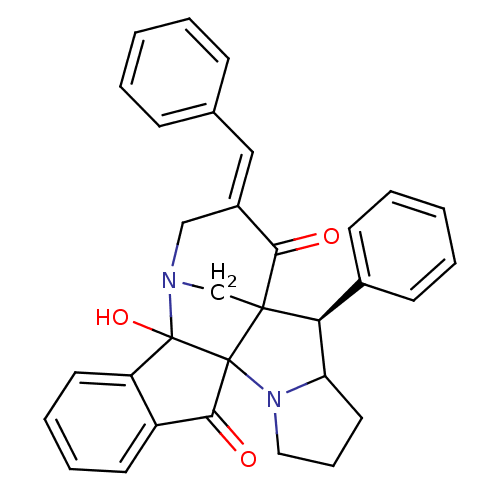
(CHEMBL1795890)Show SMILES OC12N3CC4([C@H](C5CCCN5C14C(=O)c1ccccc21)c1ccccc1)C(=O)\C(C3)=C\c1ccccc1 |r| Show InChI InChI=1S/C32H28N2O3/c35-28-23(18-21-10-3-1-4-11-21)19-33-20-30(28)27(22-12-5-2-6-13-22)26-16-9-17-34(26)31(30)29(36)24-14-7-8-15-25(24)32(31,33)37/h1-8,10-15,18,26-27,37H,9,16-17,19-20H2/b23-18+/t26?,27-,30?,31?,32?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by modified Ellman method |
Bioorg Med Chem Lett 21: 3997-4000 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.003
BindingDB Entry DOI: 10.7270/Q2TD9XPR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378876
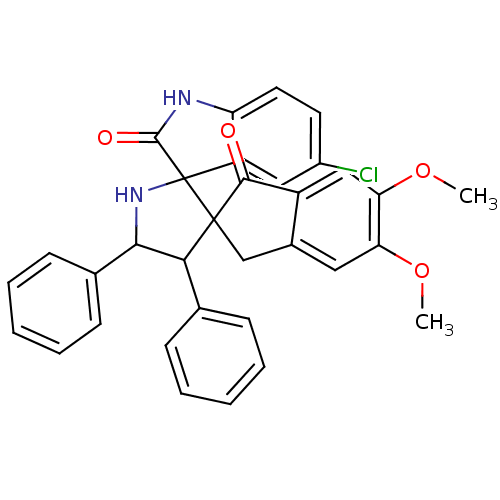
(CHEMBL1290152)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccc(Cl)cc34)c3ccccc3)c3ccccc3)C(=O)c2cc1OC Show InChI InChI=1S/C33H27ClN2O4/c1-39-26-15-21-18-32(30(37)23(21)17-27(26)40-2)28(19-9-5-3-6-10-19)29(20-11-7-4-8-12-20)36-33(32)24-16-22(34)13-14-25(24)35-31(33)38/h3-17,28-29,36H,18H2,1-2H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378873
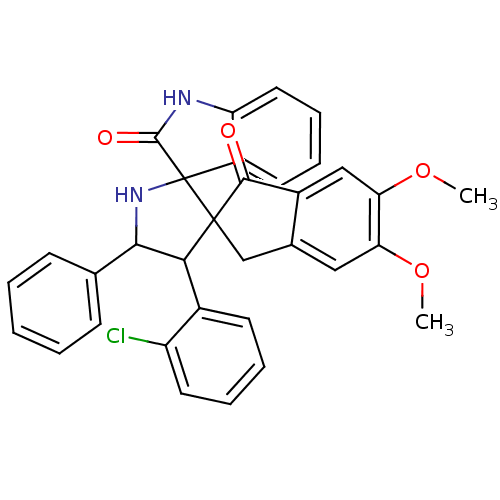
(CHEMBL1290043)Show SMILES COc1cc2CC3(C(C(NC33C(=O)Nc4ccccc34)c3ccccc3)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C33H27ClN2O4/c1-39-26-16-20-18-32(30(37)22(20)17-27(26)40-2)28(21-12-6-8-14-24(21)34)29(19-10-4-3-5-11-19)36-33(32)23-13-7-9-15-25(23)35-31(33)38/h3-17,28-29,36H,18H2,1-2H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50378869
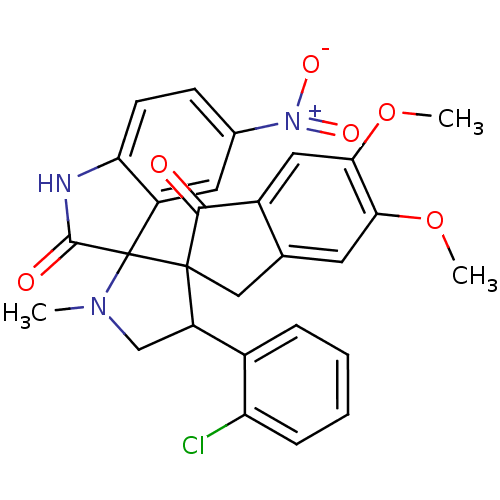
(CHEMBL1289719)Show SMILES COc1cc2CC3(C(CN(C)C33C(=O)Nc4ccc(cc34)[N+]([O-])=O)c3ccccc3Cl)C(=O)c2cc1OC Show InChI InChI=1S/C28H24ClN3O6/c1-31-14-20(17-6-4-5-7-21(17)29)27(13-15-10-23(37-2)24(38-3)12-18(15)25(27)33)28(31)19-11-16(32(35)36)8-9-22(19)30-26(28)34/h4-12,20H,13-14H2,1-3H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of human acetyl cholinesterase by Ellmann's method |
Bioorg Med Chem Lett 20: 7064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.108
BindingDB Entry DOI: 10.7270/Q2Q2417W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data