

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583383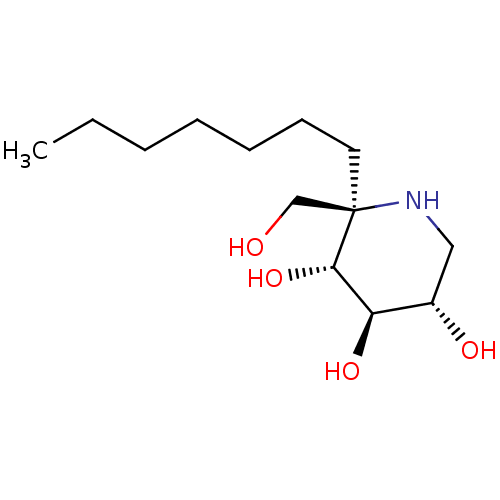 (CHEMBL5028005 | US20230339856, Compound (IIb3)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583382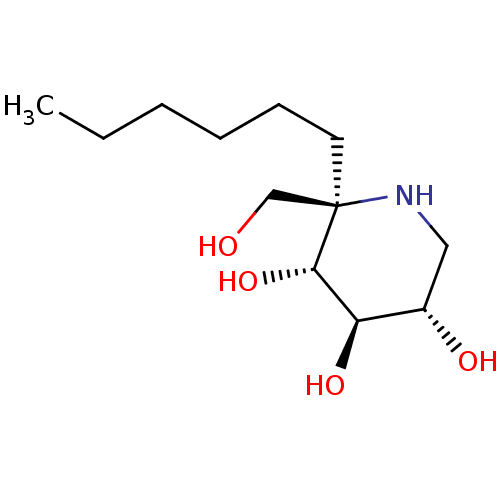 (CHEMBL5028265) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583381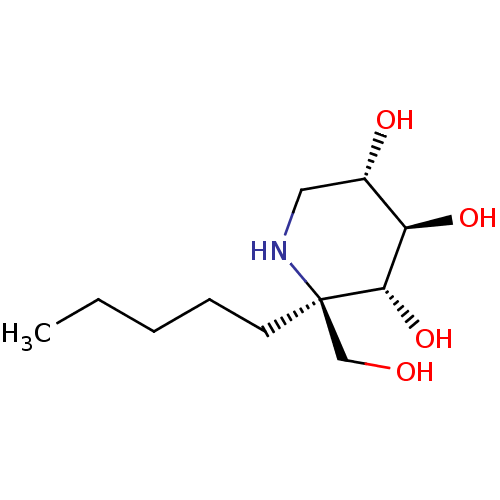 (CHEMBL5029066 | US20230339856, Compound (IIb1)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583380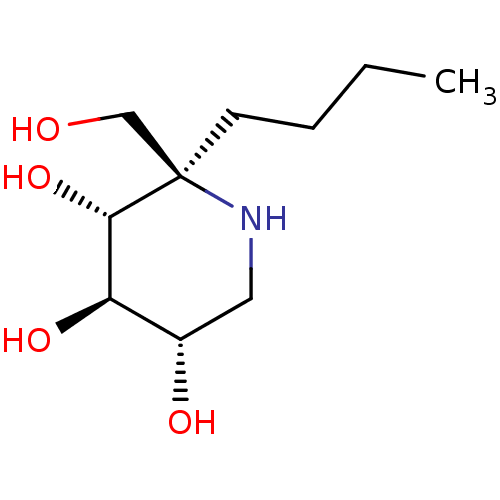 (CHEMBL5027974) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583379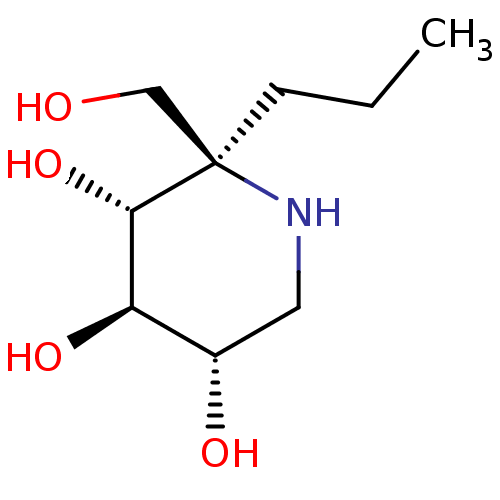 (CHEMBL5028138 | US20230339856, Compound (IIb)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335398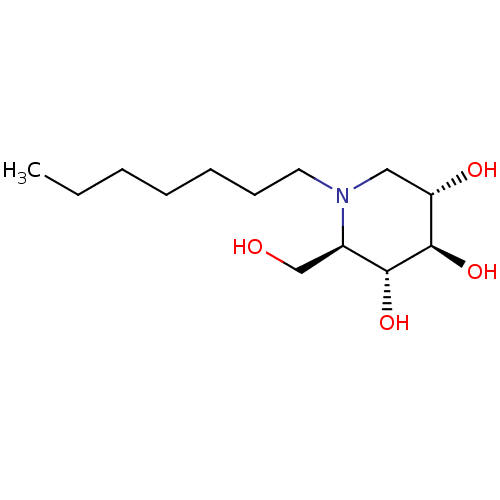 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583384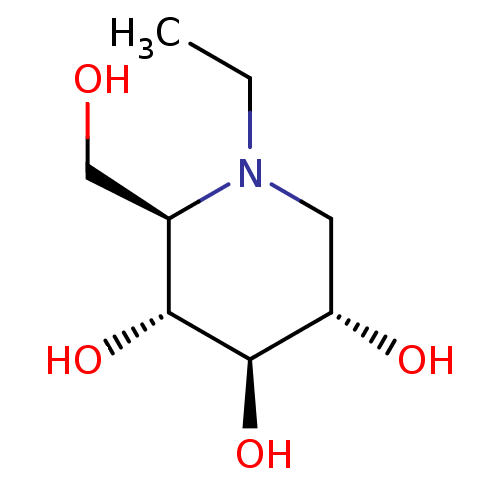 (CHEMBL5080975) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18354 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-propylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583385 (CHEMBL5028105) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583378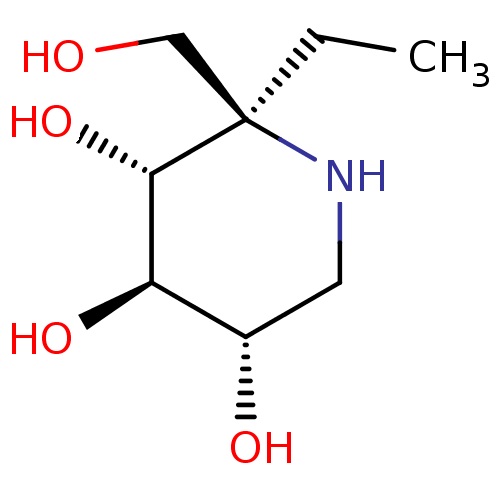 (CHEMBL5028084 | US20230339856, Compound (IIa)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583377 (CHEMBL5028072) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase (unknown origin) using PHOME as substrate measured for 1 hr by fluorescence assay | J Nat Prod 80: 1867-1875 (2017) Article DOI: 10.1021/acs.jnatprod.7b00166 BindingDB Entry DOI: 10.7270/Q20R9S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573735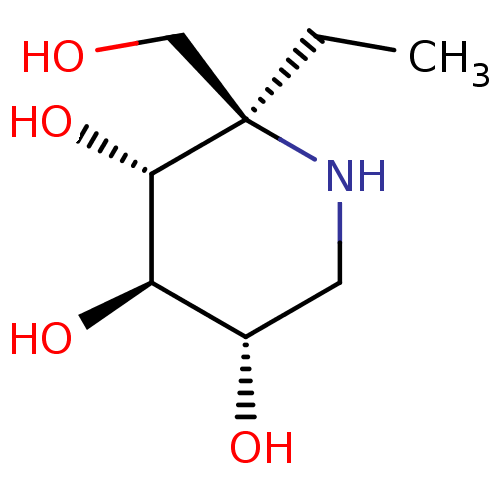 (CHEMBL4860203) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573741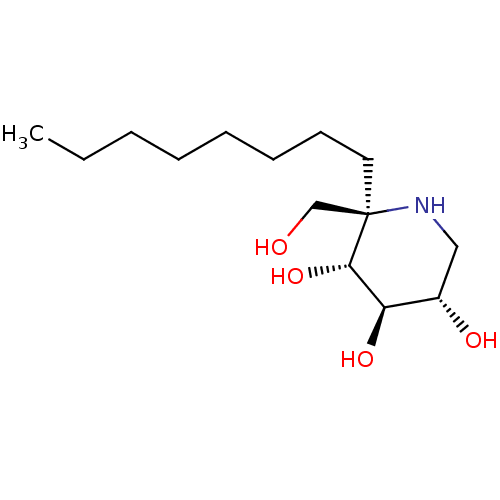 (CHEMBL4873807) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258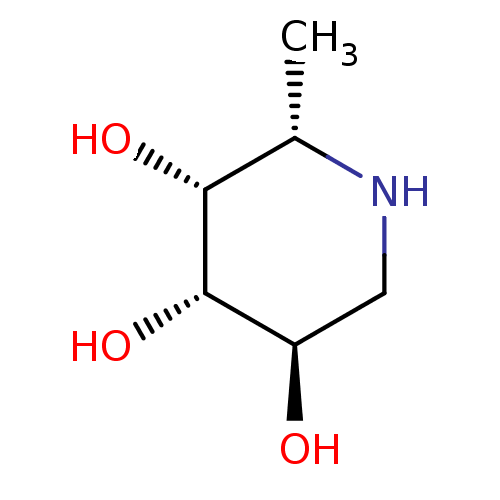 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of placental alpha-L-fucosidase (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573739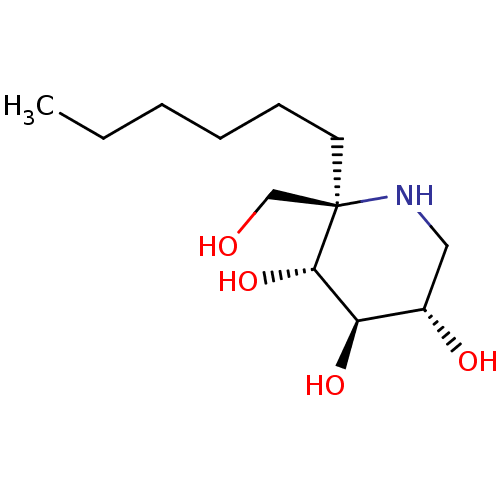 (CHEMBL4858697) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573744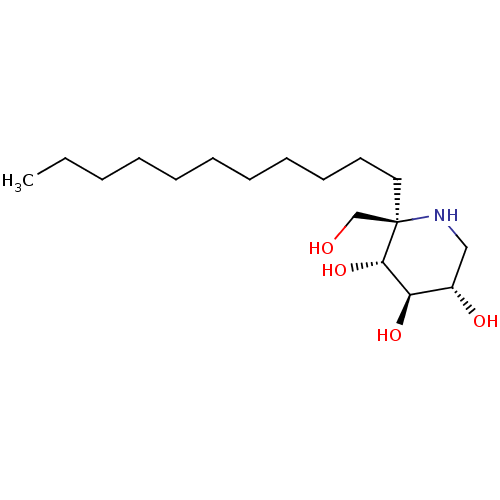 (CHEMBL4868336) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573734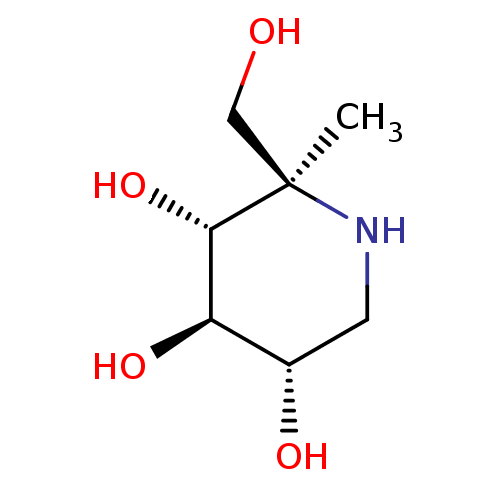 (CHEMBL4856375) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573736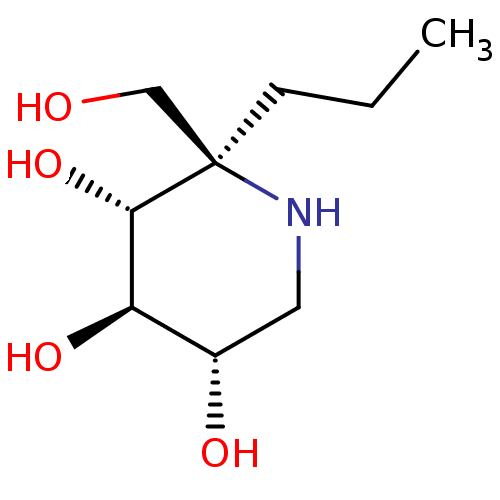 (CHEMBL4857181) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573740 (CHEMBL4855098) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573738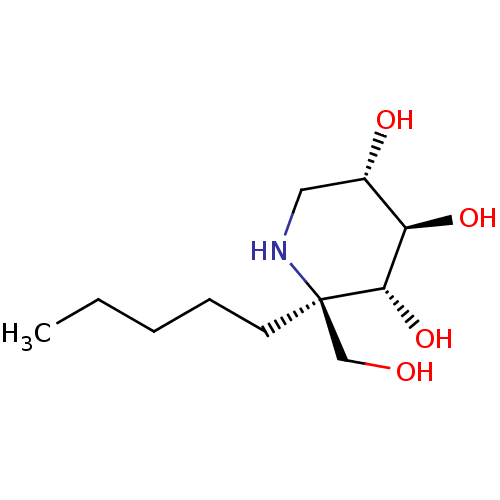 (CHEMBL4874620) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573741 (CHEMBL4873807) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50456393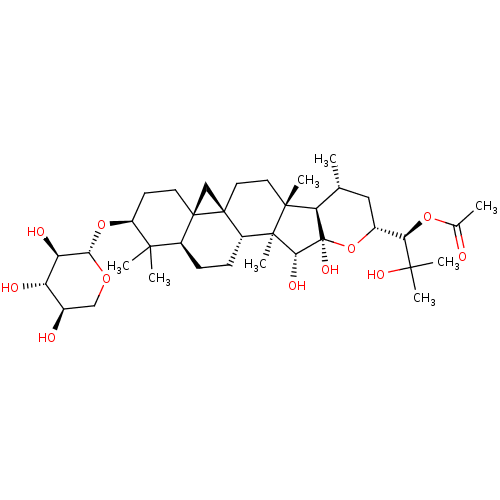 (CHEMBL4204496) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase (unknown origin) using PHOME as substrate measured for 1 hr by fluorescence assay | J Nat Prod 80: 1867-1875 (2017) Article DOI: 10.1021/acs.jnatprod.7b00166 BindingDB Entry DOI: 10.7270/Q20R9S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50456395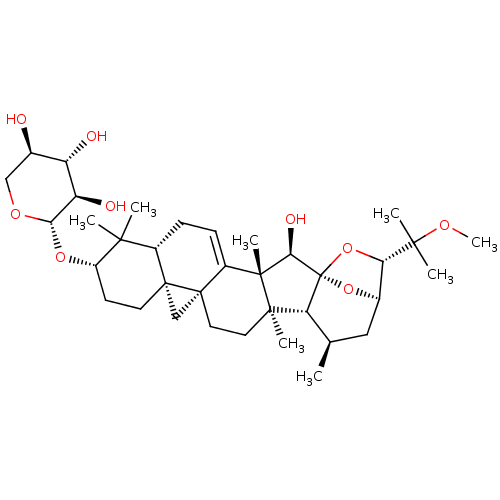 (CHEMBL4211861) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase (unknown origin) using PHOME as substrate measured for 1 hr by fluorescence assay | J Nat Prod 80: 1867-1875 (2017) Article DOI: 10.1021/acs.jnatprod.7b00166 BindingDB Entry DOI: 10.7270/Q20R9S1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573742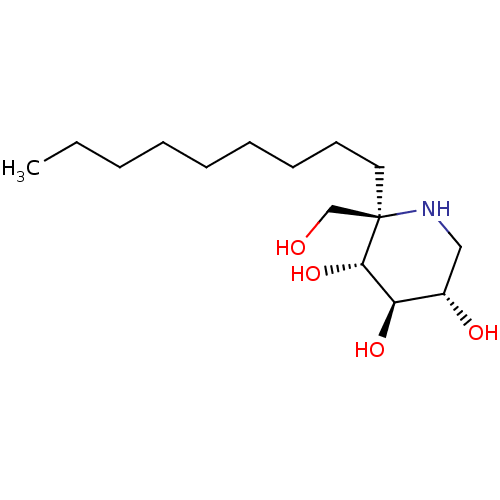 (CHEMBL4863963) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573740 (CHEMBL4855098) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573739 (CHEMBL4858697) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573737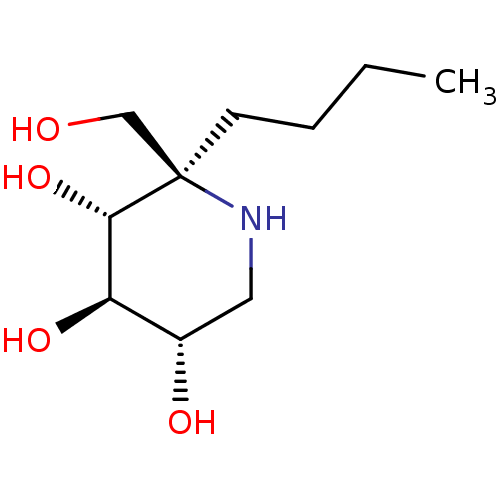 (CHEMBL4854197) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50399650 (CHEMBL2177689) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573738 (CHEMBL4874620) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573734 (CHEMBL4856375) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50375511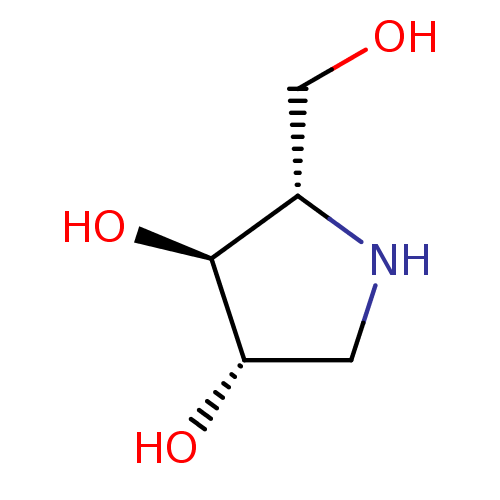 (CHEMBL406973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Sucrase (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50573734 (CHEMBL4856375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human alpha-glucosidase | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573744 (CHEMBL4868336) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573735 (CHEMBL4860203) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573745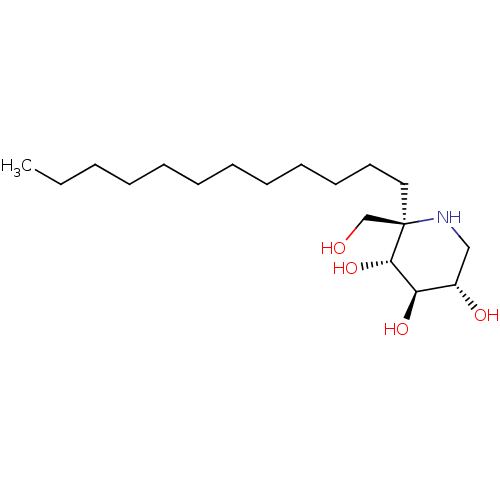 (CHEMBL4873613) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human alpha-glucosidase | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573743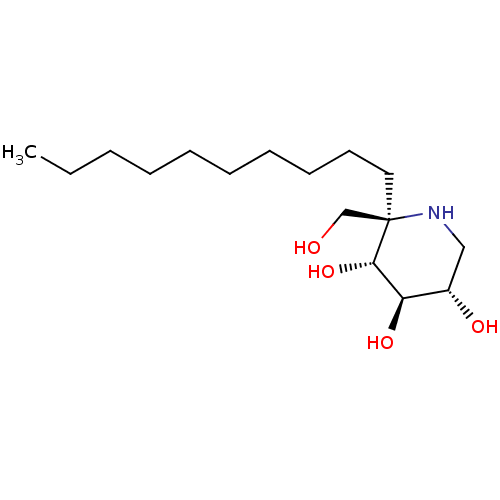 (CHEMBL4864234) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573736 (CHEMBL4857181) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573742 (CHEMBL4863963) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase (Tenebrio molitor) | BDBM50090176 (CHEMBL3581562) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of Tenebrio molitor alpha-amylase pre-incubated for 20 mins at 37 degC by bernfeld method | J Nat Prod 78: 695-704 (2015) Article DOI: 10.1021/np500866c BindingDB Entry DOI: 10.7270/Q2Q241Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50573737 (CHEMBL4854197) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat intestinal isomaltase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50573744 (CHEMBL4868336) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of coffee beans alpha-galactosidase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectromet... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113716 BindingDB Entry DOI: 10.7270/Q27W6H1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |