 Found 85 hits with Last Name = 'cardile' and Initial = 'v'
Found 85 hits with Last Name = 'cardile' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243681

(CHEMBL4069507)Show SMILES [O-][N+](=O)c1ccc(NCCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C23H28F3N3O2/c24-23(25,26)21-17-20(8-9-22(21)29(30)31)27-12-4-5-13-28-14-10-19(11-15-28)16-18-6-2-1-3-7-18/h1-3,6-9,17,19,27H,4-5,10-16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243653
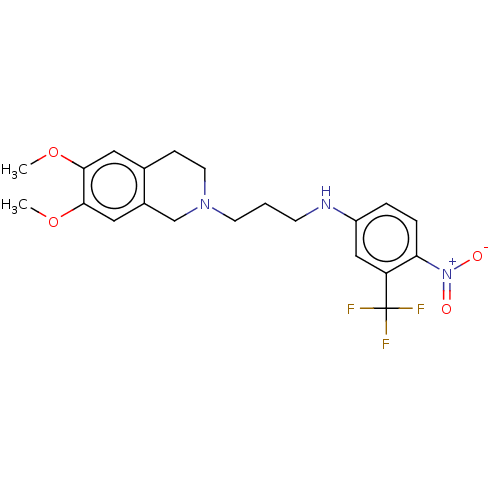
(CHEMBL4104454)Show SMILES COc1cc2CCN(CCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C21H24F3N3O4/c1-30-19-10-14-6-9-26(13-15(14)11-20(19)31-2)8-3-7-25-16-4-5-18(27(28)29)17(12-16)21(22,23)24/h4-5,10-12,25H,3,6-9,13H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]mazindol binding to the dopamine transporter. |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243681

(CHEMBL4069507)Show SMILES [O-][N+](=O)c1ccc(NCCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C23H28F3N3O2/c24-23(25,26)21-17-20(8-9-22(21)29(30)31)27-12-4-5-13-28-14-10-19(11-15-28)16-18-6-2-1-3-7-18/h1-3,6-9,17,19,27H,4-5,10-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243667

(CHEMBL4089029)Show SMILES [O-][N+](=O)c1ccc(NCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c23-22(24,25)20-16-19(7-8-21(20)28(29)30)26-11-4-12-27-13-9-18(10-14-27)15-17-5-2-1-3-6-17/h1-3,5-8,16,18,26H,4,9-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243683

(CHEMBL4084057)Show SMILES COc1cc2CCN(CCCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C22H26F3N3O4/c1-31-20-11-15-7-10-27(14-16(15)12-21(20)32-2)9-4-3-8-26-17-5-6-19(28(29)30)18(13-17)22(23,24)25/h5-6,11-13,26H,3-4,7-10,14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243684
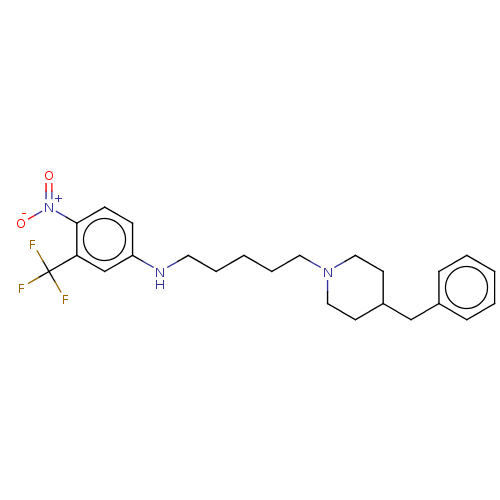
(CHEMBL4096734)Show SMILES [O-][N+](=O)c1ccc(NCCCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C24H30F3N3O2/c25-24(26,27)22-18-21(9-10-23(22)30(31)32)28-13-5-2-6-14-29-15-11-20(12-16-29)17-19-7-3-1-4-8-19/h1,3-4,7-10,18,20,28H,2,5-6,11-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243652
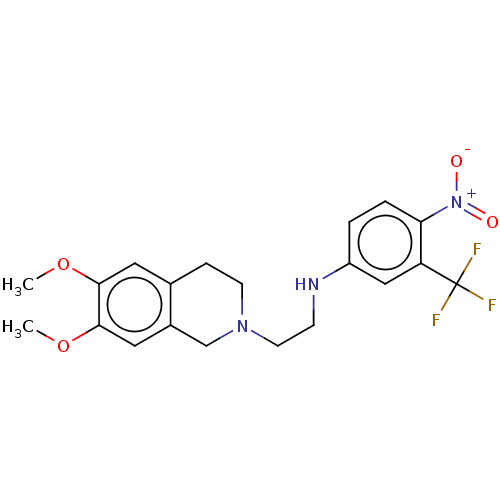
(CHEMBL4079680)Show SMILES COc1cc2CCN(CCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C20H22F3N3O4/c1-29-18-9-13-5-7-25(12-14(13)10-19(18)30-2)8-6-24-15-3-4-17(26(27)28)16(11-15)20(21,22)23/h3-4,9-11,24H,5-8,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243682

(CHEMBL4102036)Show SMILES COc1cc2CCN(CCCCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C23H28F3N3O4/c1-32-21-12-16-8-11-28(15-17(16)13-22(21)33-2)10-5-3-4-9-27-18-6-7-20(29(30)31)19(14-18)23(24,25)26/h6-7,12-14,27H,3-5,8-11,15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243683

(CHEMBL4084057)Show SMILES COc1cc2CCN(CCCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C22H26F3N3O4/c1-31-20-11-15-7-10-27(14-16(15)12-21(20)32-2)9-4-3-8-26-17-5-6-19(28(29)30)18(13-17)22(23,24)25/h5-6,11-13,26H,3-4,7-10,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to the dopamine transporter. |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243682

(CHEMBL4102036)Show SMILES COc1cc2CCN(CCCCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C23H28F3N3O4/c1-32-21-12-16-8-11-28(15-17(16)13-22(21)33-2)10-5-3-4-9-27-18-6-7-20(29(30)31)19(14-18)23(24,25)26/h6-7,12-14,27H,3-5,8-11,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243684
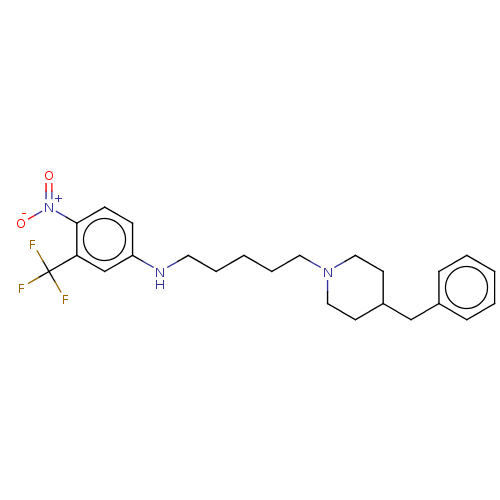
(CHEMBL4096734)Show SMILES [O-][N+](=O)c1ccc(NCCCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C24H30F3N3O2/c25-24(26,27)22-18-21(9-10-23(22)30(31)32)28-13-5-2-6-14-29-15-11-20(12-16-29)17-19-7-3-1-4-8-19/h1,3-4,7-10,18,20,28H,2,5-6,11-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243654

(CHEMBL4060828)Show SMILES [O-][N+](=O)c1ccc(NCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C21H24F3N3O2/c22-21(23,24)19-15-18(6-7-20(19)27(28)29)25-10-13-26-11-8-17(9-12-26)14-16-4-2-1-3-5-16/h1-7,15,17,25H,8-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243667

(CHEMBL4089029)Show SMILES [O-][N+](=O)c1ccc(NCCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c23-22(24,25)20-16-19(7-8-21(20)28(29)30)26-11-4-12-27-13-9-18(10-14-27)15-17-5-2-1-3-6-17/h1-3,5-8,16,18,26H,4,9-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50243654

(CHEMBL4060828)Show SMILES [O-][N+](=O)c1ccc(NCCN2CCC(Cc3ccccc3)CC2)cc1C(F)(F)F Show InChI InChI=1S/C21H24F3N3O2/c22-21(23,24)19-15-18(6-7-20(19)27(28)29)25-10-13-26-11-8-17(9-12-26)14-16-4-2-1-3-5-16/h1-7,15,17,25H,8-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from sigma 2 receptor (unknown origin) after 120 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243653

(CHEMBL4104454)Show SMILES COc1cc2CCN(CCCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C21H24F3N3O4/c1-30-19-10-14-6-9-26(13-15(14)11-20(19)31-2)8-3-7-25-16-4-5-18(27(28)29)17(12-16)21(22,23)24/h4-5,10-12,25H,3,6-9,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine uptake at dopamine transporter. |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232100
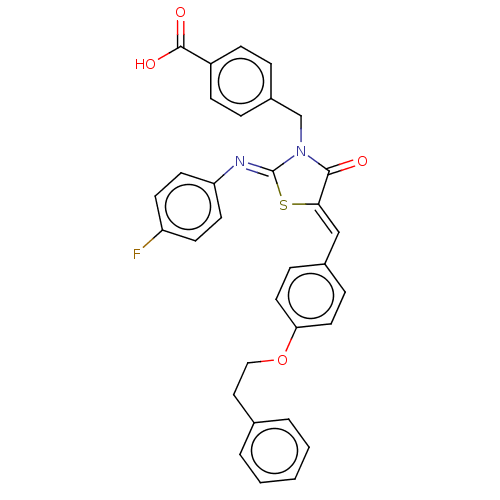
(CHEMBL4061225)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-12-14-27(15-13-26)34-32-35(21-24-6-10-25(11-7-24)31(37)38)30(36)29(40-32)20-23-8-16-28(17-9-23)39-19-18-22-4-2-1-3-5-22/h1-17,20H,18-19,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232102
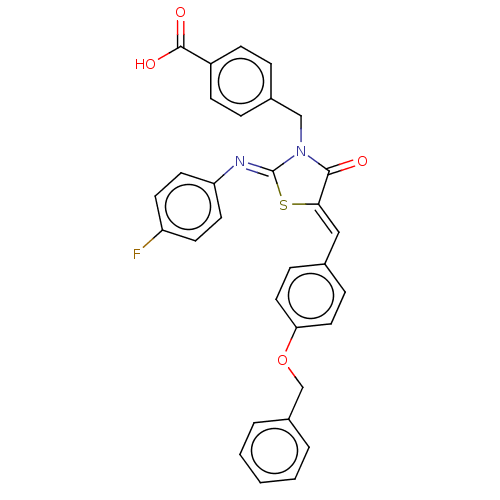
(CHEMBL4080177)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-12-14-26(15-13-25)33-31-34(19-22-6-10-24(11-7-22)30(36)37)29(35)28(39-31)18-21-8-16-27(17-9-21)38-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232101
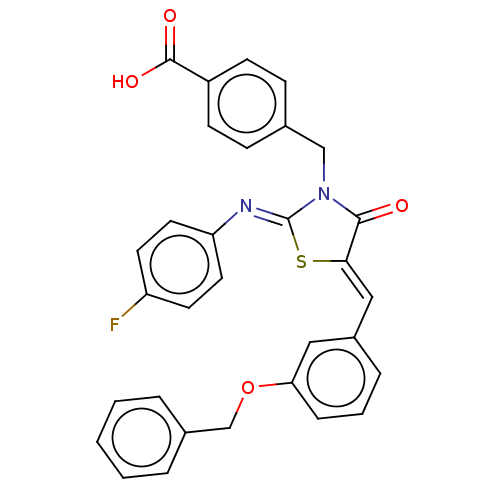
(CHEMBL4098207)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-13-15-26(16-14-25)33-31-34(19-21-9-11-24(12-10-21)30(36)37)29(35)28(39-31)18-23-7-4-8-27(17-23)38-20-22-5-2-1-3-6-22/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232103
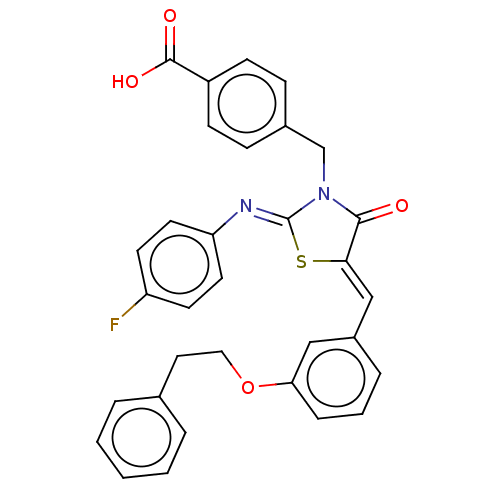
(CHEMBL4089378)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCCc3ccccc3)c2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-13-15-27(16-14-26)34-32-35(21-23-9-11-25(12-10-23)31(37)38)30(36)29(40-32)20-24-7-4-8-28(19-24)39-18-17-22-5-2-1-3-6-22/h1-16,19-20H,17-18,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50243652

(CHEMBL4079680)Show SMILES COc1cc2CCN(CCNc3ccc(c(c3)C(F)(F)F)[N+]([O-])=O)Cc2cc1OC Show InChI InChI=1S/C20H22F3N3O4/c1-29-18-9-13-5-7-25(12-14(13)10-19(18)30-2)8-6-24-15-3-4-17(26(27)28)16(11-15)20(21,22)23/h3-4,9-11,24H,5-8,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 150 mins by liquid scintillation counting method |
J Med Chem 60: 9531-9544 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00791
BindingDB Entry DOI: 10.7270/Q2NS0X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232093
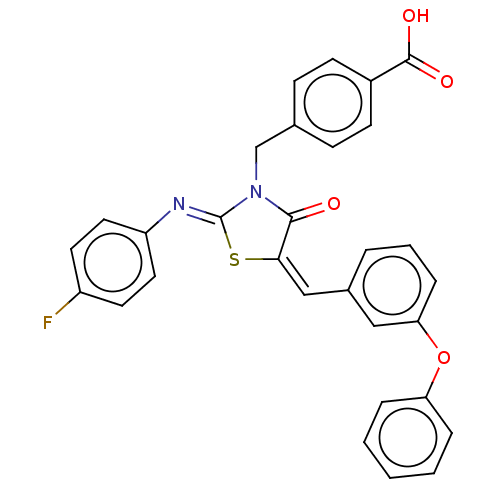
(CHEMBL4072175)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(Oc3ccccc3)c2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-13-15-24(16-14-23)32-30-33(19-20-9-11-22(12-10-20)29(35)36)28(34)27(38-30)18-21-5-4-8-26(17-21)37-25-6-2-1-3-7-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232094
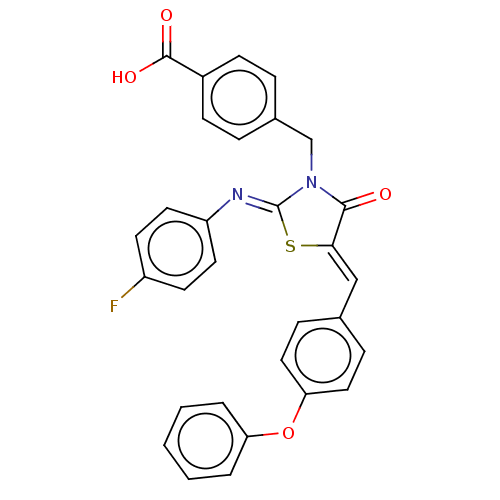
(CHEMBL4062661)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-12-14-24(15-13-23)32-30-33(19-21-6-10-22(11-7-21)29(35)36)28(34)27(38-30)18-20-8-16-26(17-9-20)37-25-4-2-1-3-5-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM24529
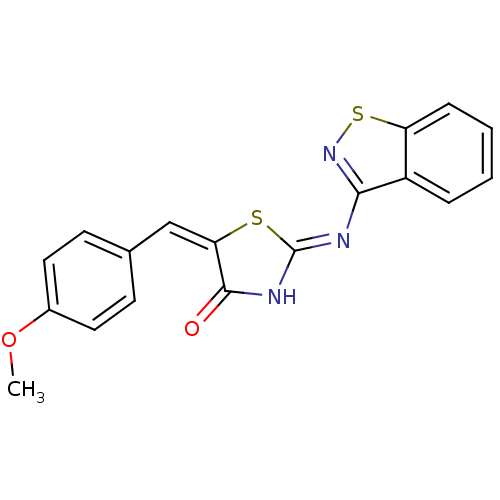
((2Z,5E)-2-(1,2-benzothiazol-3-ylimino)-5-[(4-metho...)Show SMILES COc1ccc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)cc1 Show InChI InChI=1S/C18H13N3O2S2/c1-23-12-8-6-11(7-9-12)10-15-17(22)20-18(24-15)19-16-13-4-2-3-5-14(13)25-21-16/h2-10H,1H3,(H,19,20,21,22)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Catania
| Assay Description
The MMP assay were performed evaluating the ability of compounds 1a-6a and 1b-6b to prevent the hydrolysis of the fluorescence-quenched peptide subst... |
Bioorg Chem 39: 48-52 (2011)
Article DOI: 10.1016/j.bioorg.2010.11.002
BindingDB Entry DOI: 10.7270/Q28914DM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016230
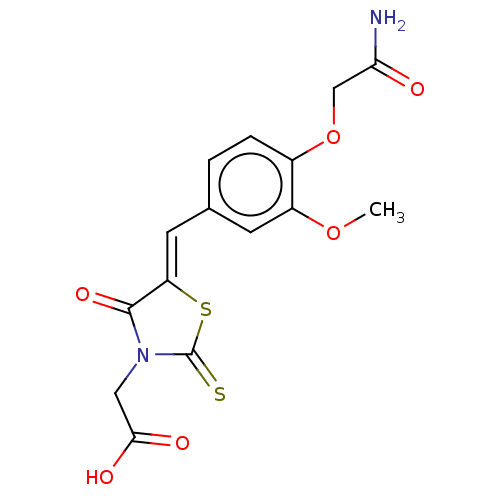
(CHEMBL3262478)Show SMILES COc1cc(\C=C2/SC(=S)N(CC(O)=O)C2=O)ccc1OCC(N)=O Show InChI InChI=1S/C15H14N2O6S2/c1-22-10-4-8(2-3-9(10)23-7-12(16)18)5-11-14(21)17(6-13(19)20)15(24)25-11/h2-5H,6-7H2,1H3,(H2,16,18)(H,19,20)/b11-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016232

(CHEMBL3262476)Show SMILES NC(=O)COc1ccc(\C=C2/SC(=S)N(CC(O)=O)C2=O)cc1 Show InChI InChI=1S/C14H12N2O5S2/c15-11(17)7-21-9-3-1-8(2-4-9)5-10-13(20)16(6-12(18)19)14(22)23-10/h1-5H,6-7H2,(H2,15,17)(H,18,19)/b10-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016233
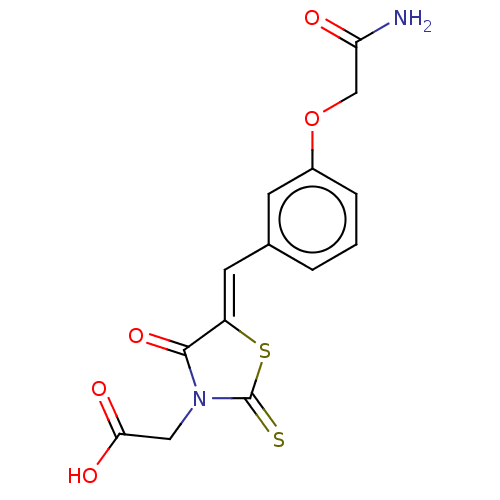
(CHEMBL3262475)Show SMILES NC(=O)COc1cccc(\C=C2/SC(=S)N(CC(O)=O)C2=O)c1 Show InChI InChI=1S/C14H12N2O5S2/c15-11(17)7-21-9-3-1-2-8(4-9)5-10-13(20)16(6-12(18)19)14(22)23-10/h1-5H,6-7H2,(H2,15,17)(H,18,19)/b10-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016231
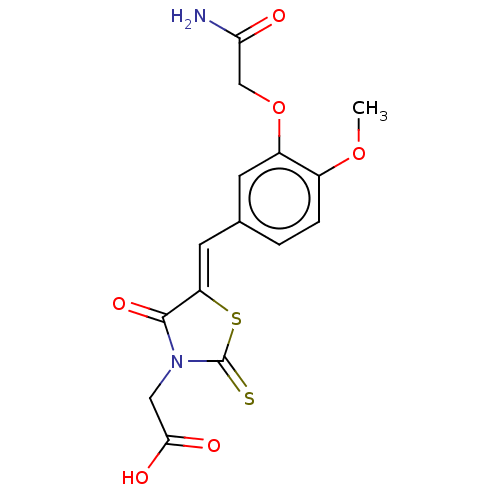
(CHEMBL3262477)Show SMILES COc1ccc(\C=C2/SC(=S)N(CC(O)=O)C2=O)cc1OCC(N)=O Show InChI InChI=1S/C15H14N2O6S2/c1-22-9-3-2-8(4-10(9)23-7-12(16)18)5-11-14(21)17(6-13(19)20)15(24)25-11/h2-5H,6-7H2,1H3,(H2,16,18)(H,19,20)/b11-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50359900
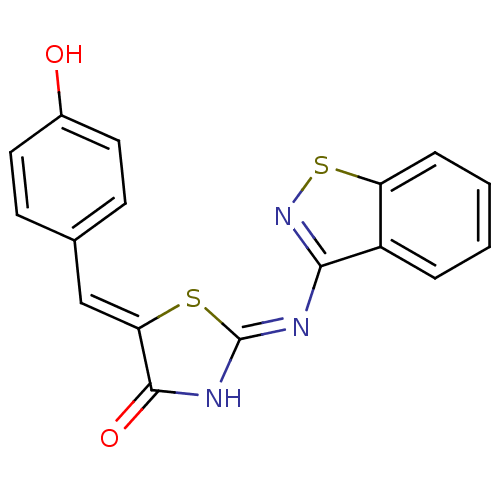
(CHEMBL260125)Show SMILES Oc1ccc(\C=C2/S\C(NC2=O)=N/c2nsc3ccccc23)cc1 Show InChI InChI=1S/C17H11N3O2S2/c21-11-7-5-10(6-8-11)9-14-16(22)19-17(23-14)18-15-12-3-1-2-4-13(12)24-20-15/h1-9,21H,(H,18,19,20,22)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured f... |
Bioorg Med Chem 23: 1551-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.002
BindingDB Entry DOI: 10.7270/Q26T0P92 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM85285
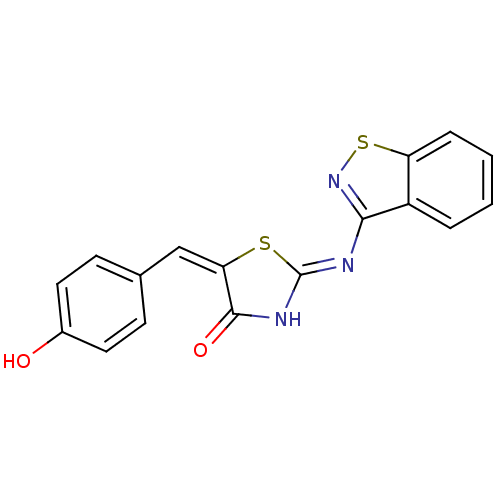
(2-(benzo[d]isothiazol-3-ylimino)-5-benzylidenethia...)Show SMILES Oc1ccc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)cc1 Show InChI InChI=1S/C17H11N3O2S2/c21-11-7-5-10(6-8-11)9-14-16(22)19-17(23-14)18-15-12-3-1-2-4-13(12)24-20-15/h1-9,21H,(H,18,19,20,22)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Catania
| Assay Description
The MMP assay were performed evaluating the ability of compounds 1a-6a and 1b-6b to prevent the hydrolysis of the fluorescence-quenched peptide subst... |
Bioorg Chem 39: 48-52 (2011)
Article DOI: 10.1016/j.bioorg.2010.11.002
BindingDB Entry DOI: 10.7270/Q28914DM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50049730
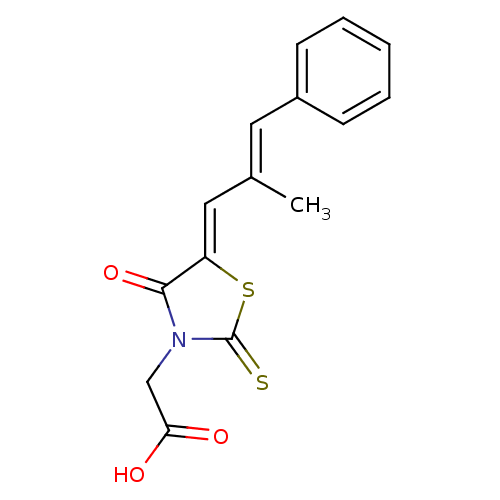
(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016234

(CHEMBL3262474)Show SMILES COc1cc(\C=C2/SC(=O)N(CC(O)=O)C2=O)ccc1OCC(N)=O Show InChI InChI=1S/C15H14N2O7S/c1-23-10-4-8(2-3-9(10)24-7-12(16)18)5-11-14(21)17(6-13(19)20)15(22)25-11/h2-5H,6-7H2,1H3,(H2,16,18)(H,19,20)/b11-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016235
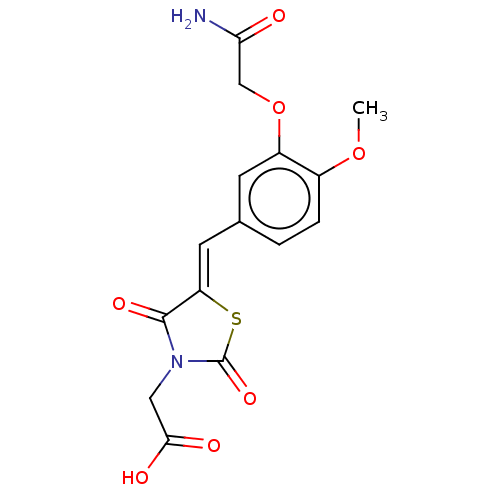
(CHEMBL3262473)Show SMILES COc1ccc(\C=C2/SC(=O)N(CC(O)=O)C2=O)cc1OCC(N)=O Show InChI InChI=1S/C15H14N2O7S/c1-23-9-3-2-8(4-10(9)24-7-12(16)18)5-11-14(21)17(6-13(19)20)15(22)25-11/h2-5H,6-7H2,1H3,(H2,16,18)(H,19,20)/b11-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016236
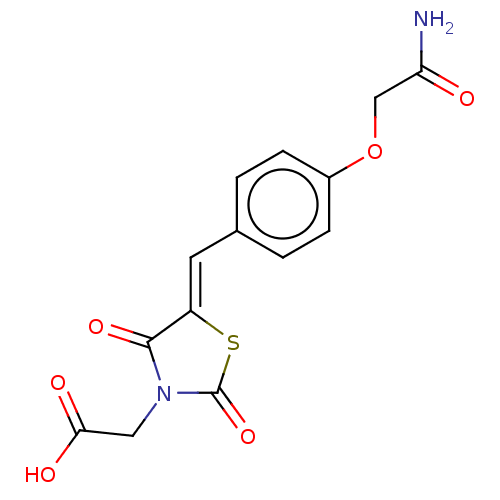
(CHEMBL3262472)Show SMILES NC(=O)COc1ccc(\C=C2/SC(=O)N(CC(O)=O)C2=O)cc1 Show InChI InChI=1S/C14H12N2O6S/c15-11(17)7-22-9-3-1-8(2-4-9)5-10-13(20)16(6-12(18)19)14(21)23-10/h1-5H,6-7H2,(H2,15,17)(H,18,19)/b10-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016237
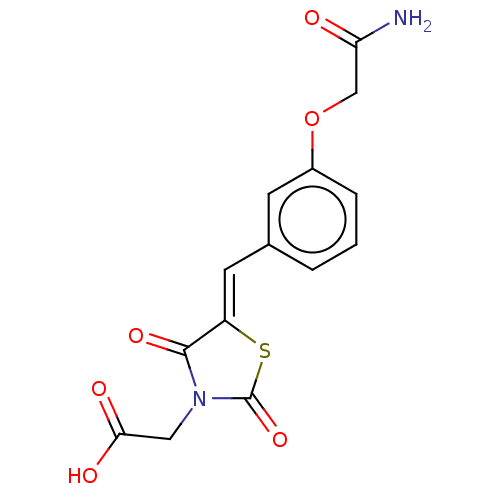
(CHEMBL3262471)Show SMILES NC(=O)COc1cccc(\C=C2/SC(=O)N(CC(O)=O)C2=O)c1 Show InChI InChI=1S/C14H12N2O6S/c15-11(17)7-22-9-3-1-2-8(4-9)5-10-13(20)16(6-12(18)19)14(21)23-10/h1-5H,6-7H2,(H2,15,17)(H,18,19)/b10-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232100

(CHEMBL4061225)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-12-14-27(15-13-26)34-32-35(21-24-6-10-25(11-7-24)31(37)38)30(36)29(40-32)20-23-8-16-28(17-9-23)39-19-18-22-4-2-1-3-5-22/h1-17,20H,18-19,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232095
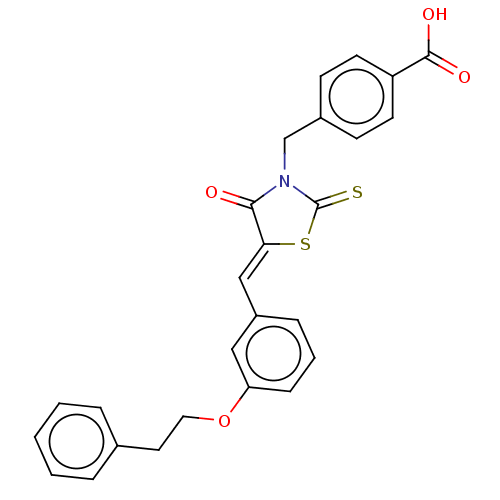
(CHEMBL4081501)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3cccc(OCCc4ccccc4)c3)C2=O)cc1 Show InChI InChI=1S/C26H21NO4S2/c28-24-23(33-26(32)27(24)17-19-9-11-21(12-10-19)25(29)30)16-20-7-4-8-22(15-20)31-14-13-18-5-2-1-3-6-18/h1-12,15-16H,13-14,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232102

(CHEMBL4080177)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-12-14-26(15-13-25)33-31-34(19-22-6-10-24(11-7-22)30(36)37)29(35)28(39-31)18-21-8-16-27(17-9-21)38-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM16312

((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...)Show InChI InChI=1S/C11H9FN2O3/c12-6-1-2-8-7(5-6)11(3-4-17-8)9(15)13-10(16)14-11/h1-2,5H,3-4H2,(H2,13,14,15,16)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50359901
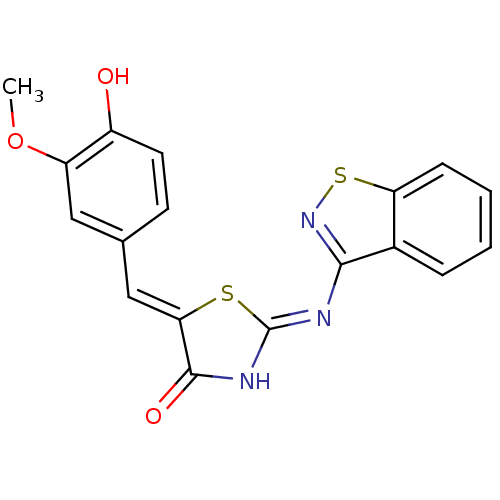
(CHEMBL260886)Show SMILES COc1cc(\C=C2/S\C(NC2=O)=N/c2nsc3ccccc23)ccc1O Show InChI InChI=1S/C18H13N3O3S2/c1-24-13-8-10(6-7-12(13)22)9-15-17(23)20-18(25-15)19-16-11-4-2-3-5-14(11)26-21-16/h2-9,22H,1H3,(H,19,20,21,23)/b15-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured fo... |
Bioorg Med Chem 23: 1551-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.002
BindingDB Entry DOI: 10.7270/Q26T0P92 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50276752
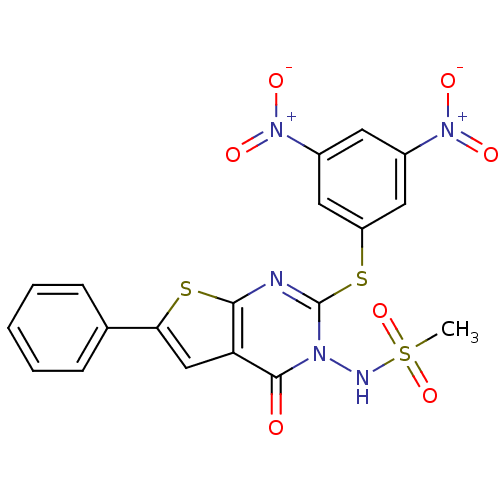
(CHEMBL460015 | N-[2-[(2,4-dinitrophenyl)thio]-4-ox...)Show SMILES CS(=O)(=O)Nn1c(Sc2cc(cc(c2)[N+]([O-])=O)[N+]([O-])=O)nc2sc(cc2c1=O)-c1ccccc1 Show InChI InChI=1S/C19H13N5O7S3/c1-34(30,31)21-22-18(25)15-10-16(11-5-3-2-4-6-11)33-17(15)20-19(22)32-14-8-12(23(26)27)7-13(9-14)24(28)29/h2-10,21H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human whole blood assessed as inhibition of PGE2 production by radioimmunoassay |
Bioorg Med Chem 17: 1991-6 (2009)
Article DOI: 10.1016/j.bmc.2009.01.029
BindingDB Entry DOI: 10.7270/Q24B316J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232096
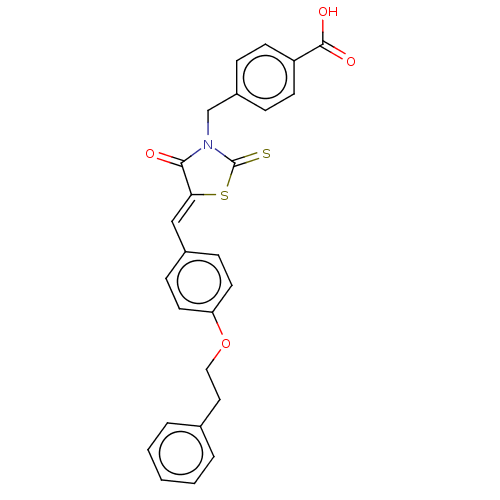
(CHEMBL4099520)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO4S2/c28-24-23(33-26(32)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)31-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232101

(CHEMBL4098207)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-13-15-26(16-14-25)33-31-34(19-21-9-11-24(12-10-21)30(36)37)29(35)28(39-31)18-23-7-4-8-27(17-23)38-20-22-5-2-1-3-6-22/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232097
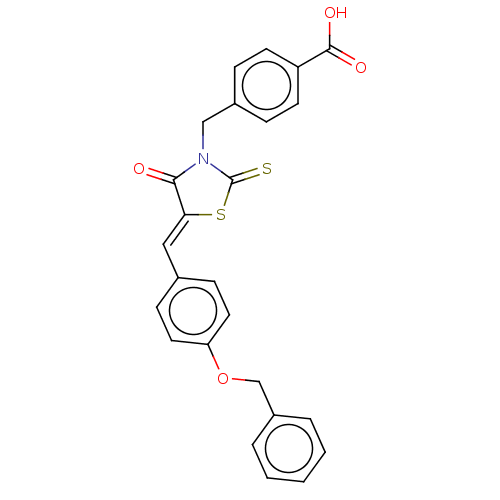
(CHEMBL4091784)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3ccc(OCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C25H19NO4S2/c27-23-22(32-25(31)26(23)15-18-6-10-20(11-7-18)24(28)29)14-17-8-12-21(13-9-17)30-16-19-4-2-1-3-5-19/h1-14H,15-16H2,(H,28,29)/b22-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50359898

(CHEMBL412064)Show SMILES Clc1cccc(\C=C2/S\C(NC2=O)=N/c2nsc3ccccc23)c1 Show InChI InChI=1S/C17H10ClN3OS2/c18-11-5-3-4-10(8-11)9-14-16(22)20-17(23-14)19-15-12-6-1-2-7-13(12)24-21-15/h1-9H,(H,19,20,21,22)/b14-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured fo... |
Bioorg Med Chem 23: 1551-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.002
BindingDB Entry DOI: 10.7270/Q26T0P92 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232094

(CHEMBL4062661)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-12-14-24(15-13-23)32-30-33(19-21-6-10-22(11-7-21)29(35)36)28(34)27(38-30)18-20-8-16-26(17-9-20)37-25-4-2-1-3-5-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232104
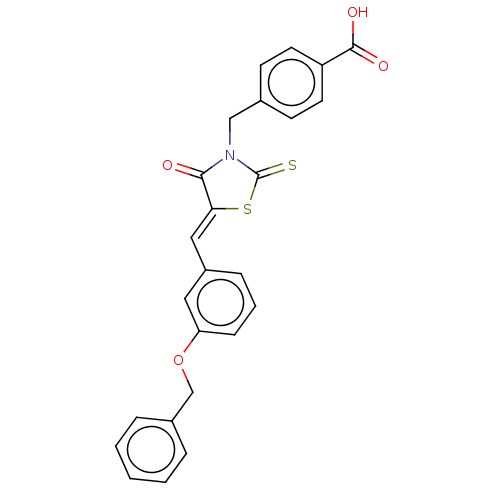
(CHEMBL4070916)Show SMILES OC(=O)c1ccc(CN2C(=S)S\C(=C/c3cccc(OCc4ccccc4)c3)C2=O)cc1 Show InChI InChI=1S/C25H19NO4S2/c27-23-22(32-25(31)26(23)15-17-9-11-20(12-10-17)24(28)29)14-19-7-4-8-21(13-19)30-16-18-5-2-1-3-6-18/h1-14H,15-16H2,(H,28,29)/b22-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PTP1B using pNPP as substrate by spectrophotometric method |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50276751

(2-[(3-Amino-4-oxo-3,4-dihydroquinazolin-2-yl)thio]...)Show InChI InChI=1S/C11H11N3O3S/c1-6(10(16)17)18-11-13-8-5-3-2-4-7(8)9(15)14(11)12/h2-6H,12H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in LPS-stimulated human whole blood assessed as inhibition of PGE2 production by radioimmunoassay |
Bioorg Med Chem 17: 1991-6 (2009)
Article DOI: 10.1016/j.bmc.2009.01.029
BindingDB Entry DOI: 10.7270/Q24B316J |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50359897
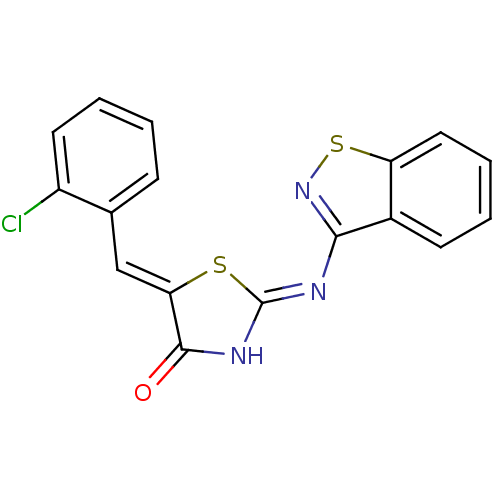
(CHEMBL264164)Show InChI InChI=1S/C17H10ClN3OS2/c18-12-7-3-1-5-10(12)9-14-16(22)20-17(23-14)19-15-11-6-2-4-8-13(11)24-21-15/h1-9H,(H,19,20,21,22)/b14-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain (unknown origin) assessed as reduction in hydrolysis of Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate measured fo... |
Bioorg Med Chem 23: 1551-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.002
BindingDB Entry DOI: 10.7270/Q26T0P92 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data