 Found 547 hits with Last Name = 'garaj' and Initial = 'v'
Found 547 hits with Last Name = 'garaj' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153970

(4-(4,6-Diethoxy-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C13H17N5O4S/c1-3-21-12-16-11(17-13(18-12)22-4-2)15-9-5-7-10(8-6-9)23(14,19)20/h5-8H,3-4H2,1-2H3,(H2,14,19,20)(H,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153971
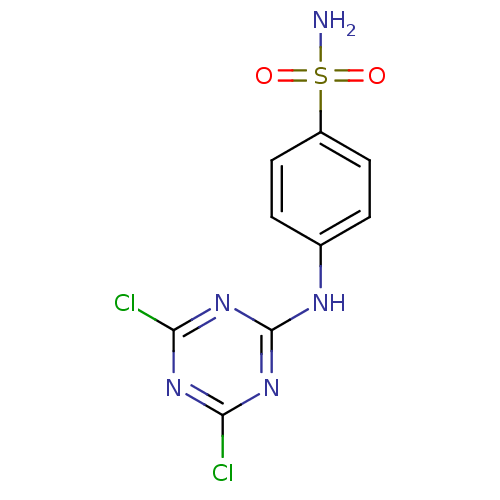
(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153971

(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153964

(4-((4,6-diethoxy-1,3,5-triazin-2-ylamino)methyl)be...)Show InChI InChI=1S/C14H19N5O4S/c1-3-22-13-17-12(18-14(19-13)23-4-2)16-9-10-5-7-11(8-6-10)24(15,20)21/h5-8H,3-4,9H2,1-2H3,(H2,15,20,21)(H,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50153971

(4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenes...)Show InChI InChI=1S/C9H7Cl2N5O2S/c10-7-14-8(11)16-9(15-7)13-5-1-3-6(4-2-5)19(12,17)18/h1-4H,(H2,12,17,18)(H,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349847

(CHEMBL1813208)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCCC(O)=O)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C15H19N7O6S/c16-29(27,28)10-3-1-9(2-4-10)19-15-21-13(17-7-5-11(23)24)20-14(22-15)18-8-6-12(25)26/h1-4H,5-8H2,(H,23,24)(H,25,26)(H2,16,27,28)(H3,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349847

(CHEMBL1813208)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCCC(O)=O)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C15H19N7O6S/c16-29(27,28)10-3-1-9(2-4-10)19-15-21-13(17-7-5-11(23)24)20-14(22-15)18-8-6-12(25)26/h1-4H,5-8H2,(H,23,24)(H,25,26)(H2,16,27,28)(H3,17,18,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349848
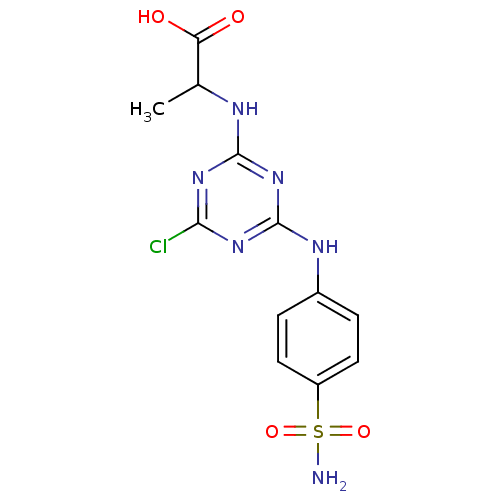
(CHEMBL1813209)Show SMILES CC(Nc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1)C(O)=O Show InChI InChI=1S/C12H13ClN6O4S/c1-6(9(20)21)15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,22)23/h2-6H,1H3,(H,20,21)(H2,14,22,23)(H2,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167355
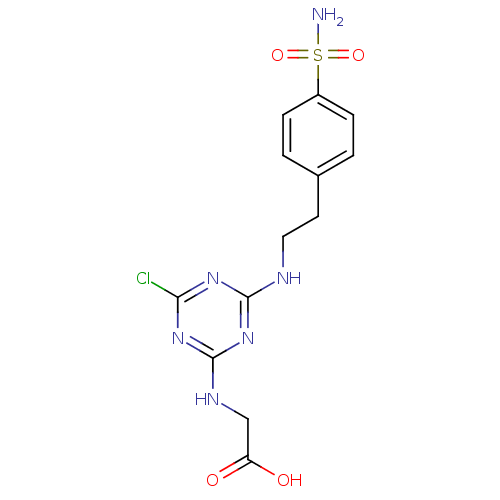
(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167353

(4-[2-(4,6-Diamino-[1,3,5]triazin-2-ylamino)-ethyl]...)Show InChI InChI=1S/C11H15N7O2S/c12-9-16-10(13)18-11(17-9)15-6-5-7-1-3-8(4-2-7)21(14,19)20/h1-4H,5-6H2,(H2,14,19,20)(H5,12,13,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167355

(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153969
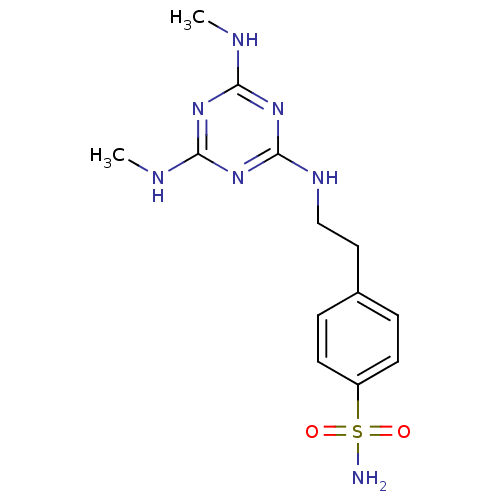
(4-[2-(4,6-Bis-methylamino-[1,3,5]triazin-2-ylamino...)Show InChI InChI=1S/C13H19N7O2S/c1-15-11-18-12(16-2)20-13(19-11)17-8-7-9-3-5-10(6-4-9)23(14,21)22/h3-6H,7-8H2,1-2H3,(H2,14,21,22)(H3,15,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167337
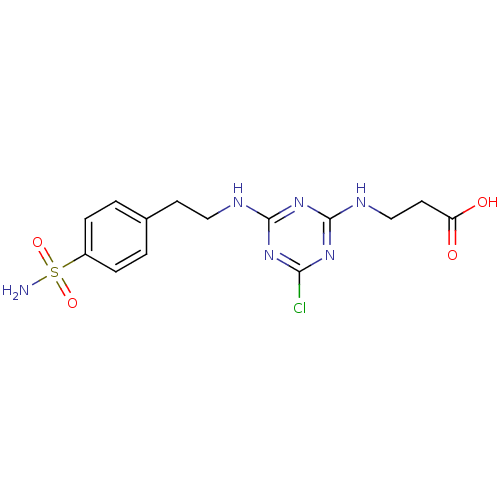
(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167361
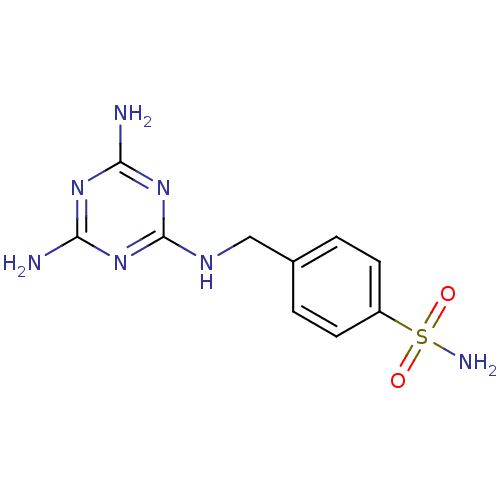
(4-[(4,6-Diamino-[1,3,5]triazin-2-ylamino)-methyl]-...)Show InChI InChI=1S/C10H13N7O2S/c11-8-15-9(12)17-10(16-8)14-5-6-1-3-7(4-2-6)20(13,18)19/h1-4H,5H2,(H2,13,18,19)(H5,11,12,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50153976
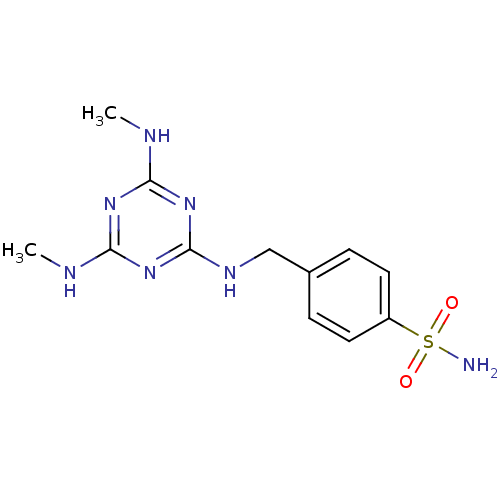
(4-[(4,6-Bis-methylamino-[1,3,5]triazin-2-ylamino)-...)Show InChI InChI=1S/C12H17N7O2S/c1-14-10-17-11(15-2)19-12(18-10)16-7-8-3-5-9(6-4-8)22(13,20)21/h3-6H,7H2,1-2H3,(H2,13,20,21)(H3,14,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167350

(4-[(4,6-Bis-dimethylamino-[1,3,5]triazin-2-ylamino...)Show SMILES CN(C)c1nc(NCc2ccc(cc2)S(N)(=O)=O)nc(n1)N(C)C Show InChI InChI=1S/C14H21N7O2S/c1-20(2)13-17-12(18-14(19-13)21(3)4)16-9-10-5-7-11(8-6-10)24(15,22)23/h5-8H,9H2,1-4H3,(H2,15,22,23)(H,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50349858
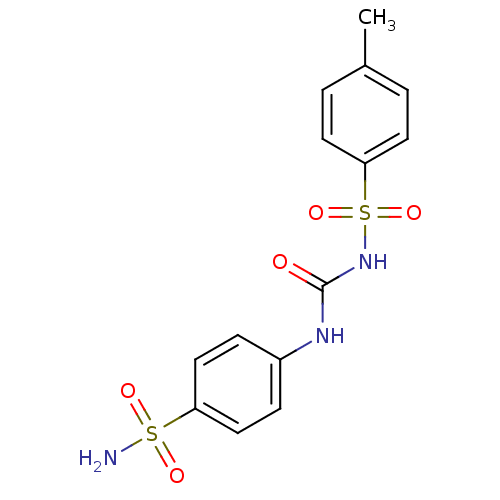
(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167341
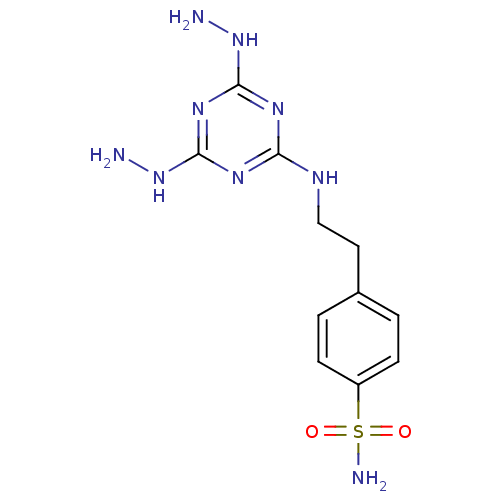
(4-[2-(4,6-Dihydrazino-[1,3,5]triazin-2-ylamino)-et...)Show InChI InChI=1S/C11H17N9O2S/c12-19-10-16-9(17-11(18-10)20-13)15-6-5-7-1-3-8(4-2-7)23(14,21)22/h1-4H,5-6,12-13H2,(H2,14,21,22)(H3,15,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167330
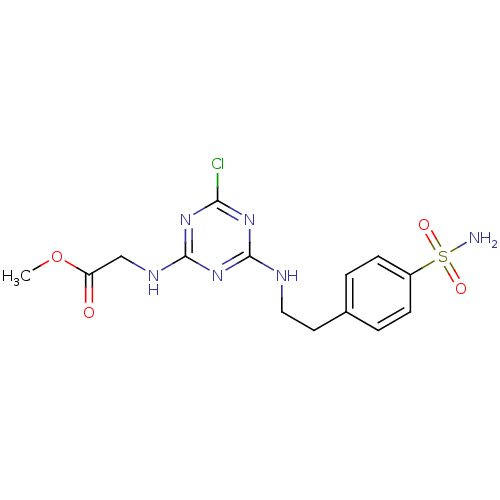
(CHEMBL190733 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES COC(=O)CNc1nc(Cl)nc(NCCc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C14H17ClN6O4S/c1-25-11(22)8-18-14-20-12(15)19-13(21-14)17-7-6-9-2-4-10(5-3-9)26(16,23)24/h2-5H,6-8H2,1H3,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167357
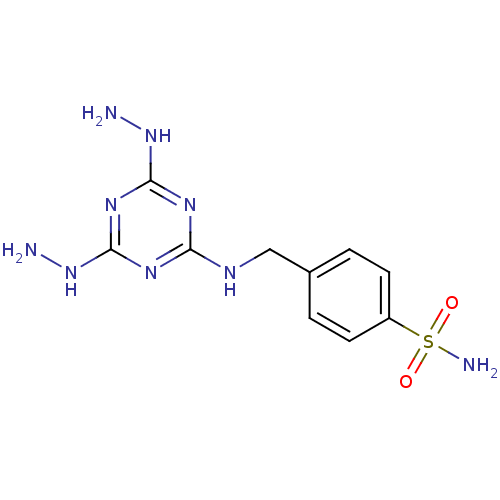
(4-[(4,6-Dihydrazino-[1,3,5]triazin-2-ylamino)-meth...)Show InChI InChI=1S/C10H15N9O2S/c11-18-9-15-8(16-10(17-9)19-12)14-5-6-1-3-7(4-2-6)22(13,20)21/h1-4H,5,11-12H2,(H2,13,20,21)(H3,14,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167330

(CHEMBL190733 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES COC(=O)CNc1nc(Cl)nc(NCCc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C14H17ClN6O4S/c1-25-11(22)8-18-14-20-12(15)19-13(21-14)17-7-6-9-2-4-10(5-3-9)26(16,23)24/h2-5H,6-8H2,1H3,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167349
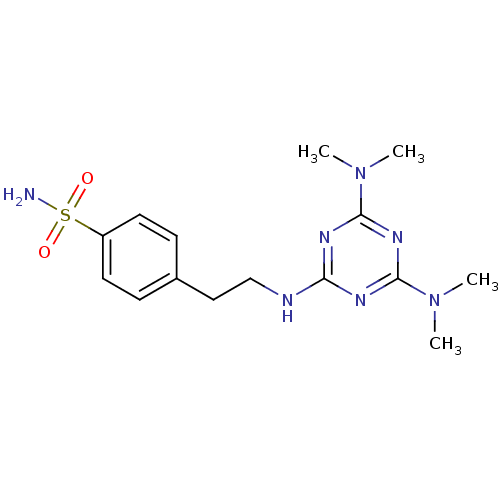
(4-[2-(4,6-Bis-dimethylamino-[1,3,5]triazin-2-ylami...)Show SMILES CN(C)c1nc(NCCc2ccc(cc2)S(N)(=O)=O)nc(n1)N(C)C Show InChI InChI=1S/C15H23N7O2S/c1-21(2)14-18-13(19-15(20-14)22(3)4)17-10-9-11-5-7-12(8-6-11)25(16,23)24/h5-8H,9-10H2,1-4H3,(H2,16,23,24)(H,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167348
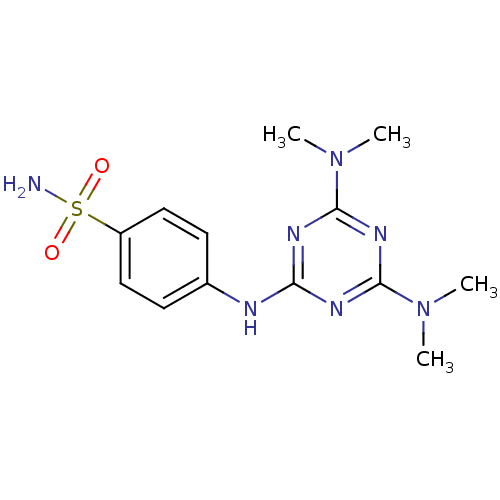
(4-(4,6-Bis-dimethylamino-[1,3,5]triazin-2-ylamino)...)Show SMILES CN(C)c1nc(Nc2ccc(cc2)S(N)(=O)=O)nc(n1)N(C)C Show InChI InChI=1S/C13H19N7O2S/c1-19(2)12-16-11(17-13(18-12)20(3)4)15-9-5-7-10(8-6-9)23(14,21)22/h5-8H,1-4H3,(H2,14,21,22)(H,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167343
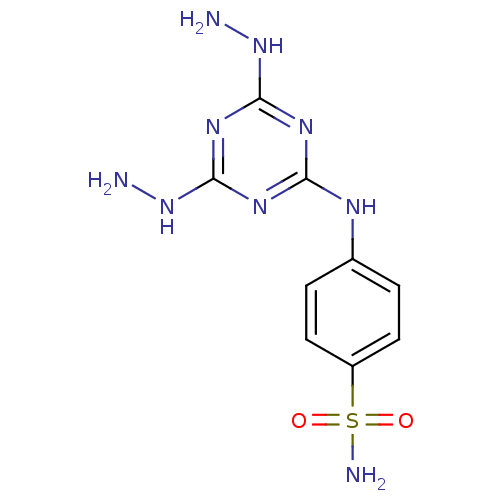
(4-(4,6-Dihydrazino-[1,3,5]triazin-2-ylamino)-benze...)Show InChI InChI=1S/C9H13N9O2S/c10-17-8-14-7(15-9(16-8)18-11)13-5-1-3-6(4-2-5)21(12,19)20/h1-4H,10-11H2,(H2,12,19,20)(H3,13,14,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349858

(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167337

(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167327
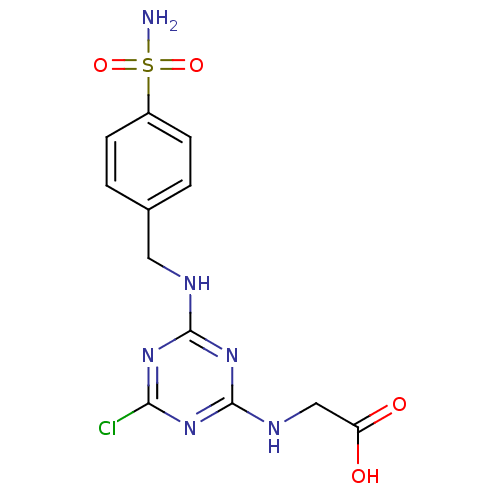
(CHEMBL191045 | [4-Chloro-6-(4-sulfamoyl-benzylamin...)Show SMILES NS(=O)(=O)c1ccc(CNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C12H13ClN6O4S/c13-10-17-11(19-12(18-10)16-6-9(20)21)15-5-7-1-3-8(4-2-7)24(14,22)23/h1-4H,5-6H2,(H,20,21)(H2,14,22,23)(H2,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM10882
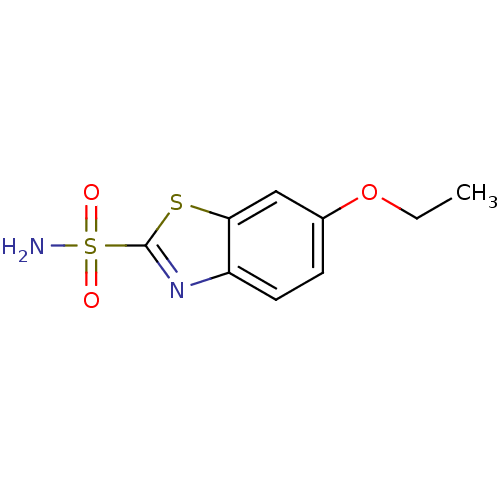
(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167329
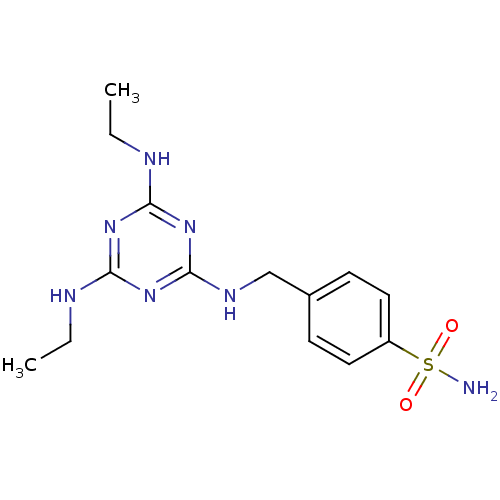
(4-[(4,6-Bis-ethylamino-[1,3,5]triazin-2-ylamino)-m...)Show InChI InChI=1S/C14H21N7O2S/c1-3-16-12-19-13(17-4-2)21-14(20-12)18-9-10-5-7-11(8-6-10)24(15,22)23/h5-8H,3-4,9H2,1-2H3,(H2,15,22,23)(H3,16,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167355

(CHEMBL189526 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15ClN6O4S/c14-11-18-12(20-13(19-11)17-7-10(21)22)16-6-5-8-1-3-9(4-2-8)25(15,23)24/h1-4H,5-7H2,(H,21,22)(H2,15,23,24)(H2,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167342

(4-[2-(4,6-Bis-ethylamino-[1,3,5]triazin-2-ylamino)...)Show InChI InChI=1S/C15H23N7O2S/c1-3-17-13-20-14(18-4-2)22-15(21-13)19-10-9-11-5-7-12(8-6-11)25(16,23)24/h5-8H,3-4,9-10H2,1-2H3,(H2,16,23,24)(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167330

(CHEMBL190733 | {4-Chloro-6-[2-(4-sulfamoyl-phenyl)...)Show SMILES COC(=O)CNc1nc(Cl)nc(NCCc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C14H17ClN6O4S/c1-25-11(22)8-18-14-20-12(15)19-13(21-14)17-7-6-9-2-4-10(5-3-9)26(16,23)24/h2-5H,6-8H2,1H3,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885
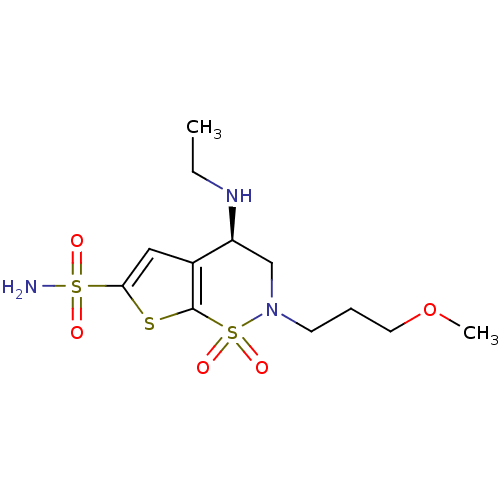
((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 14: 5427-33 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.087
BindingDB Entry DOI: 10.7270/Q2XD12DQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167337

(3-{4-Chloro-6-[2-(4-sulfamoyl-phenyl)-ethylamino]-...)Show SMILES NS(=O)(=O)c1ccc(CCNc2nc(Cl)nc(NCCC(O)=O)n2)cc1 Show InChI InChI=1S/C14H17ClN6O4S/c15-12-19-13(21-14(20-12)18-8-6-11(22)23)17-7-5-9-1-3-10(4-2-9)26(16,24)25/h1-4H,5-8H2,(H,22,23)(H2,16,24,25)(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349850

(CHEMBL1738787)Show InChI InChI=1S/C11H13ClN6O3S/c12-9-16-10(14-5-6-19)18-11(17-9)15-7-1-3-8(4-2-7)22(13,20)21/h1-4,19H,5-6H2,(H2,13,20,21)(H2,14,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349858

(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 13
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 13 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349848

(CHEMBL1813209)Show SMILES CC(Nc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1)C(O)=O Show InChI InChI=1S/C12H13ClN6O4S/c1-6(9(20)21)15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,22)23/h2-6H,1H3,(H,20,21)(H2,14,22,23)(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167325

(4-(4,6-Bis-diethylamino-[1,3,5]triazin-2-ylamino)-...)Show SMILES CCN(CC)c1nc(Nc2ccc(cc2)S(N)(=O)=O)nc(n1)N(CC)CC Show InChI InChI=1S/C17H27N7O2S/c1-5-23(6-2)16-20-15(21-17(22-16)24(7-3)8-4)19-13-9-11-14(12-10-13)27(18,25)26/h9-12H,5-8H2,1-4H3,(H2,18,25,26)(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349859
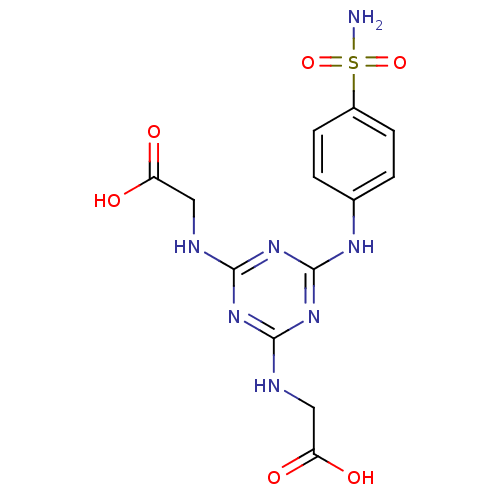
(CHEMBL1813206)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCC(O)=O)nc(NCC(O)=O)n2)cc1 Show InChI InChI=1S/C13H15N7O6S/c14-27(25,26)8-3-1-7(2-4-8)17-13-19-11(15-5-9(21)22)18-12(20-13)16-6-10(23)24/h1-4H,5-6H2,(H,21,22)(H,23,24)(H2,14,25,26)(H3,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167359
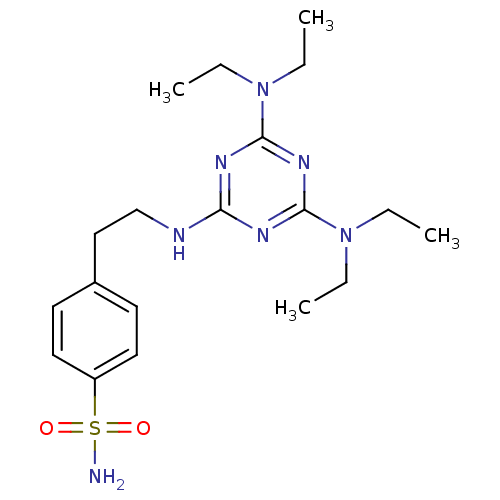
(4-[2-(4,6-Bis-diethylamino-[1,3,5]triazin-2-ylamin...)Show SMILES CCN(CC)c1nc(NCCc2ccc(cc2)S(N)(=O)=O)nc(n1)N(CC)CC Show InChI InChI=1S/C19H31N7O2S/c1-5-25(6-2)18-22-17(23-19(24-18)26(7-3)8-4)21-14-13-15-9-11-16(12-10-15)29(20,27)28/h9-12H,5-8,13-14H2,1-4H3,(H2,20,27,28)(H,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3102-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.056
BindingDB Entry DOI: 10.7270/Q2NP256X |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349846
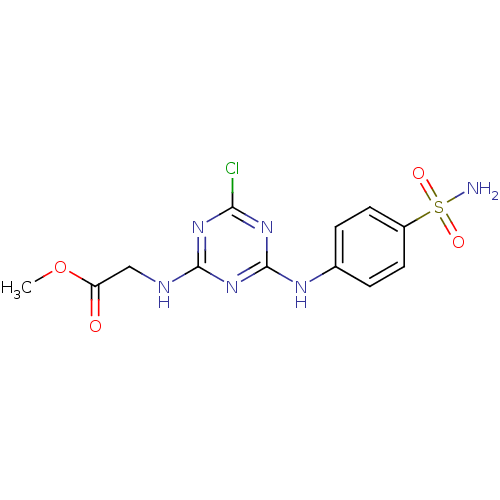
(CHEMBL1738792)Show SMILES COC(=O)CNc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C12H13ClN6O4S/c1-23-9(20)6-15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,21)22/h2-5H,6H2,1H3,(H2,14,21,22)(H2,15,16,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50349846

(CHEMBL1738792)Show SMILES COC(=O)CNc1nc(Cl)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C12H13ClN6O4S/c1-23-9(20)6-15-11-17-10(13)18-12(19-11)16-7-2-4-8(5-3-7)24(14,21)22/h2-5H,6H2,1H3,(H2,14,21,22)(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 14
(Homo sapiens (Human)) | BDBM50349845
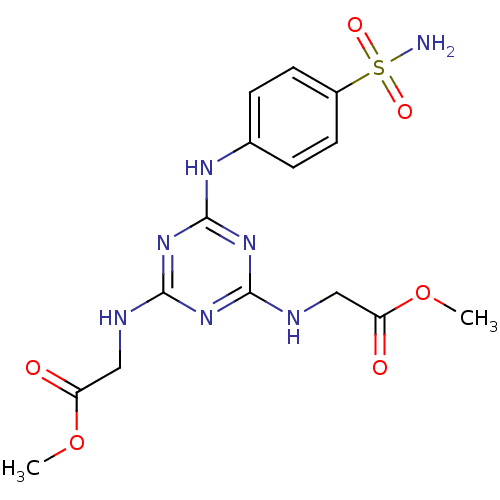
(CHEMBL1813207)Show SMILES COC(=O)CNc1nc(NCC(=O)OC)nc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C15H19N7O6S/c1-27-11(23)7-17-13-20-14(18-8-12(24)28-2)22-15(21-13)19-9-3-5-10(6-4-9)29(16,25)26/h3-6H,7-8H2,1-2H3,(H2,16,25,26)(H3,17,18,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50349858

(CHEMBL78755)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H15N3O5S2/c1-10-2-6-13(7-3-10)24(21,22)17-14(18)16-11-4-8-12(9-5-11)23(15,19)20/h2-9H,1H3,(H2,15,19,20)(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration method |
Bioorg Med Chem 19: 3105-19 (2011)
Article DOI: 10.1016/j.bmc.2011.04.005
BindingDB Entry DOI: 10.7270/Q2FF3SQ6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data