 Found 82 hits with Last Name = 'hook' and Initial = 'v'
Found 82 hits with Last Name = 'hook' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
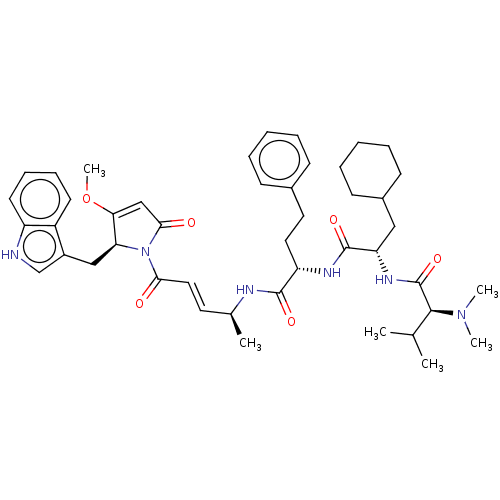
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505559

(CHEMBL4441303)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:48| Show InChI InChI=1S/C40H62N4O7/c1-12-28(8)37(43(9)10)40(49)51-34(22-27(6)7)39(48)42-31(20-25(2)3)38(47)41-30(23-29-16-14-13-15-17-29)18-19-35(45)44-32(21-26(4)5)33(50-11)24-36(44)46/h13-19,24-28,30-32,34,37H,12,20-23H2,1-11H3,(H,41,47)(H,42,48)/b19-18+/t28-,30+,31-,32-,34-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.149 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505555

(CHEMBL4447348)Show SMILES COC1=CC(=O)N([C@H]1CC(C)C)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](C(C)C)N(C)C |r,t:2| Show InChI InChI=1S/C39H60N4O7/c1-24(2)19-30(41-38(47)33(21-26(5)6)50-39(48)36(27(7)8)42(9)10)37(46)40-29(22-28-15-13-12-14-16-28)17-18-34(44)43-31(20-25(3)4)32(49-11)23-35(43)45/h12-18,23-27,29-31,33,36H,19-22H2,1-11H3,(H,40,46)(H,41,47)/b18-17+/t29-,30+,31+,33+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.304 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505557
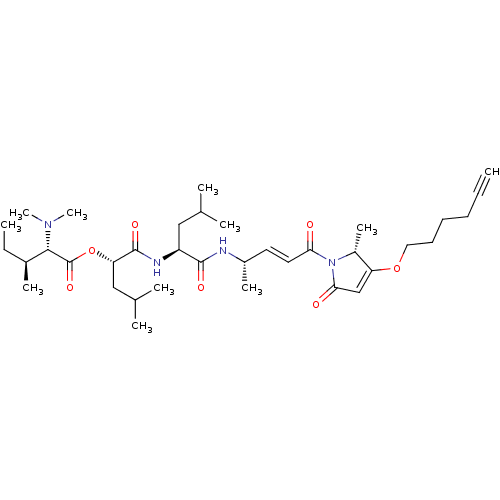
(CHEMBL4527930)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@H](C)C(OCCCCC#C)=CC1=O |r,c:43| Show InChI InChI=1S/C36H58N4O7/c1-12-14-15-16-19-46-29-22-32(42)40(27(29)9)31(41)18-17-26(8)37-34(43)28(20-23(3)4)38-35(44)30(21-24(5)6)47-36(45)33(39(10)11)25(7)13-2/h1,17-18,22-28,30,33H,13-16,19-21H2,2-11H3,(H,37,43)(H,38,44)/b18-17+/t25-,26-,27+,28-,30-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505568
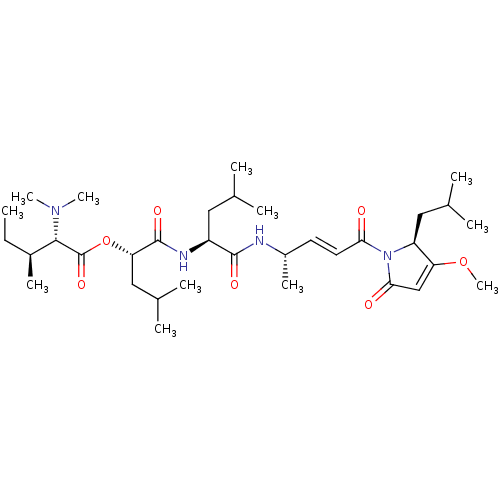
(CHEMBL4482954)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:41| Show InChI InChI=1S/C34H58N4O7/c1-13-23(8)31(37(10)11)34(43)45-28(18-22(6)7)33(42)36-25(16-20(2)3)32(41)35-24(9)14-15-29(39)38-26(17-21(4)5)27(44-12)19-30(38)40/h14-15,19-26,28,31H,13,16-18H2,1-12H3,(H,35,41)(H,36,42)/b15-14+/t23-,24-,25-,26-,28-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505555

(CHEMBL4447348)Show SMILES COC1=CC(=O)N([C@H]1CC(C)C)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](C(C)C)N(C)C |r,t:2| Show InChI InChI=1S/C39H60N4O7/c1-24(2)19-30(41-38(47)33(21-26(5)6)50-39(48)36(27(7)8)42(9)10)37(46)40-29(22-28-15-13-12-14-16-28)17-18-34(44)43-31(20-25(3)4)32(49-11)23-35(43)45/h12-18,23-27,29-31,33,36H,19-22H2,1-11H3,(H,40,46)(H,41,47)/b18-17+/t29-,30+,31+,33+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.632 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505562
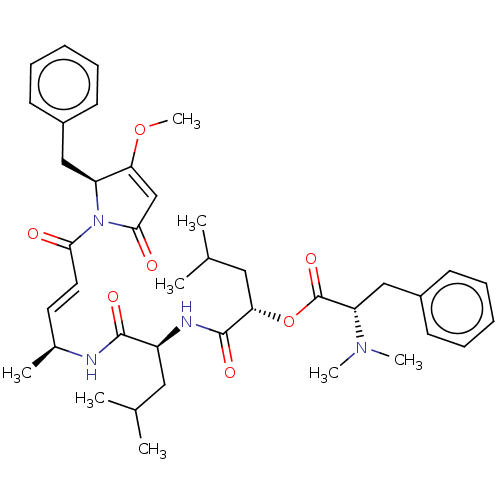
(CHEMBL4445744)Show SMILES COC1=CC(=O)N([C@H]1Cc1ccccc1)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C40H54N4O7/c1-26(2)21-31(42-39(48)35(22-27(3)4)51-40(49)33(43(6)7)24-30-17-13-10-14-18-30)38(47)41-28(5)19-20-36(45)44-32(34(50-8)25-37(44)46)23-29-15-11-9-12-16-29/h9-20,25-28,31-33,35H,21-24H2,1-8H3,(H,41,47)(H,42,48)/b20-19+/t28-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505554

(CHEMBL4564747)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](Cc2ccccc2)C(OC)=CC1=O |r,c:45| Show InChI InChI=1S/C37H56N4O7/c1-11-25(6)34(40(8)9)37(46)48-31(20-24(4)5)36(45)39-28(19-23(2)3)35(44)38-26(7)17-18-32(42)41-29(30(47-10)22-33(41)43)21-27-15-13-12-14-16-27/h12-18,22-26,28-29,31,34H,11,19-21H2,1-10H3,(H,38,44)(H,39,45)/b18-17+/t25-,26-,28-,29-,31-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505557

(CHEMBL4527930)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@H](C)C(OCCCCC#C)=CC1=O |r,c:43| Show InChI InChI=1S/C36H58N4O7/c1-12-14-15-16-19-46-29-22-32(42)40(27(29)9)31(41)18-17-26(8)37-34(43)28(20-23(3)4)38-35(44)30(21-24(5)6)47-36(45)33(39(10)11)25(7)13-2/h1,17-18,22-28,30,33H,13-16,19-21H2,2-11H3,(H,37,43)(H,38,44)/b18-17+/t25-,26-,27+,28-,30-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505563

(CHEMBL4527329)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:45| Show InChI InChI=1S/C37H56N4O7/c1-11-25(6)34(40(8)9)37(46)48-31(20-24(4)5)36(45)39-29(19-23(2)3)35(44)38-28(21-27-15-13-12-14-16-27)17-18-32(42)41-26(7)30(47-10)22-33(41)43/h12-18,22-26,28-29,31,34H,11,19-21H2,1-10H3,(H,38,44)(H,39,45)/b18-17+/t25-,26-,28+,29-,31-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505566

(CHEMBL4582558)Show SMILES COC1=CC(=O)N([C@@H]1Cc1ccccc1)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C46H58N4O7/c1-31(2)25-37(48-45(54)41(26-32(3)4)57-46(55)39(49(5)6)29-35-21-15-10-16-22-35)44(53)47-36(27-33-17-11-8-12-18-33)23-24-42(51)50-38(40(56-7)30-43(50)52)28-34-19-13-9-14-20-34/h8-24,30-32,36-39,41H,25-29H2,1-7H3,(H,47,53)(H,48,54)/b24-23+/t36-,37+,38-,39+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505565
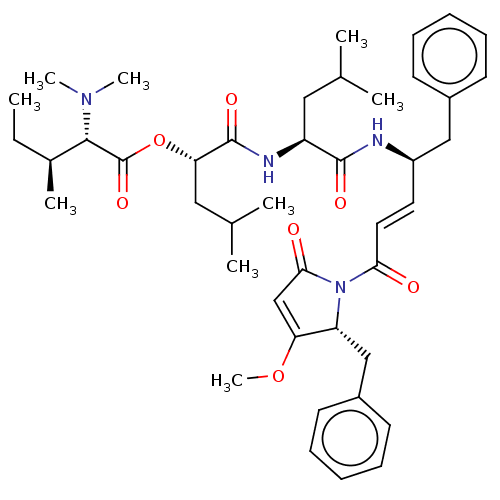
(CHEMBL4474668)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@H](Cc2ccccc2)C(OC)=CC1=O |r,c:52| Show InChI InChI=1S/C43H60N4O7/c1-10-30(6)40(46(7)8)43(52)54-37(24-29(4)5)42(51)45-34(23-28(2)3)41(50)44-33(25-31-17-13-11-14-18-31)21-22-38(48)47-35(36(53-9)27-39(47)49)26-32-19-15-12-16-20-32/h11-22,27-30,33-35,37,40H,10,23-26H2,1-9H3,(H,44,50)(H,45,51)/b22-21+/t30-,33+,34-,35+,37-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505559

(CHEMBL4441303)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:48| Show InChI InChI=1S/C40H62N4O7/c1-12-28(8)37(43(9)10)40(49)51-34(22-27(6)7)39(48)42-31(20-25(2)3)38(47)41-30(23-29-16-14-13-15-17-29)18-19-35(45)44-32(21-26(4)5)33(50-11)24-36(44)46/h13-19,24-28,30-32,34,37H,12,20-23H2,1-11H3,(H,41,47)(H,42,48)/b19-18+/t28-,30+,31-,32-,34-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505564

(CHEMBL4527867)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@@H](Cc2ccccc2)C(OC)=CC1=O |r,c:52| Show InChI InChI=1S/C43H60N4O7/c1-10-30(6)40(46(7)8)43(52)54-37(24-29(4)5)42(51)45-34(23-28(2)3)41(50)44-33(25-31-17-13-11-14-18-31)21-22-38(48)47-35(36(53-9)27-39(47)49)26-32-19-15-12-16-20-32/h11-22,27-30,33-35,37,40H,10,23-26H2,1-9H3,(H,44,50)(H,45,51)/b22-21+/t30-,33+,34-,35-,37-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505561
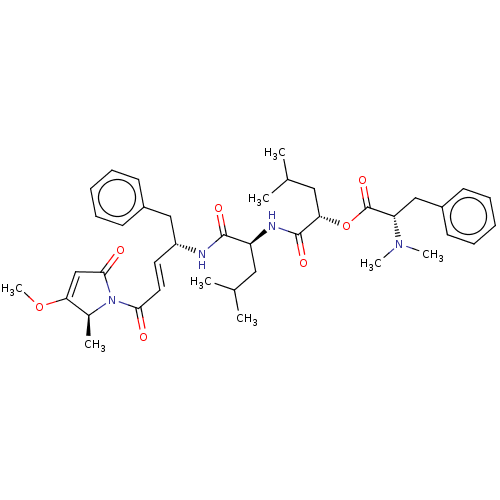
(CHEMBL4475810)Show SMILES COC1=CC(=O)N([C@H]1C)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C40H54N4O7/c1-26(2)21-32(42-39(48)35(22-27(3)4)51-40(49)33(43(6)7)24-30-17-13-10-14-18-30)38(47)41-31(23-29-15-11-9-12-16-29)19-20-36(45)44-28(5)34(50-8)25-37(44)46/h9-20,25-28,31-33,35H,21-24H2,1-8H3,(H,41,47)(H,42,48)/b20-19+/t28-,31+,32-,33-,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505560
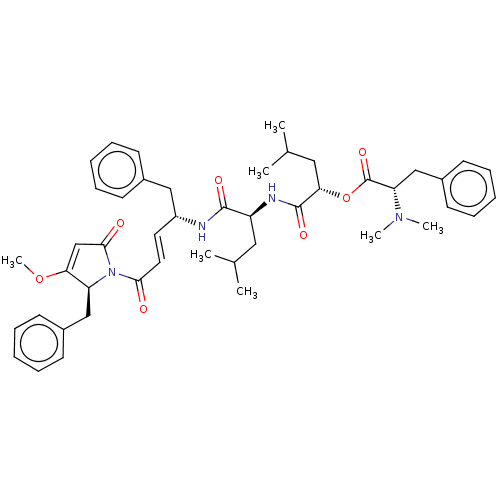
(CHEMBL4471760)Show SMILES COC1=CC(=O)N([C@H]1Cc1ccccc1)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C46H58N4O7/c1-31(2)25-37(48-45(54)41(26-32(3)4)57-46(55)39(49(5)6)29-35-21-15-10-16-22-35)44(53)47-36(27-33-17-11-8-12-18-33)23-24-42(51)50-38(40(56-7)30-43(50)52)28-34-19-13-9-14-20-34/h8-24,30-32,36-39,41H,25-29H2,1-7H3,(H,47,53)(H,48,54)/b24-23+/t36-,37+,38+,39+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505562

(CHEMBL4445744)Show SMILES COC1=CC(=O)N([C@H]1Cc1ccccc1)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C40H54N4O7/c1-26(2)21-31(42-39(48)35(22-27(3)4)51-40(49)33(43(6)7)24-30-17-13-10-14-18-30)38(47)41-28(5)19-20-36(45)44-32(34(50-8)25-37(44)46)23-29-15-11-9-12-16-29/h9-20,25-28,31-33,35H,21-24H2,1-8H3,(H,41,47)(H,42,48)/b20-19+/t28-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505560

(CHEMBL4471760)Show SMILES COC1=CC(=O)N([C@H]1Cc1ccccc1)C(=O)\C=C\[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](Cc1ccccc1)N(C)C |r,t:2| Show InChI InChI=1S/C46H58N4O7/c1-31(2)25-37(48-45(54)41(26-32(3)4)57-46(55)39(49(5)6)29-35-21-15-10-16-22-35)44(53)47-36(27-33-17-11-8-12-18-33)23-24-42(51)50-38(40(56-7)30-43(50)52)28-34-19-13-9-14-20-34/h8-24,30-32,36-39,41H,25-29H2,1-7H3,(H,47,53)(H,48,54)/b24-23+/t36-,37+,38+,39+,41+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505565

(CHEMBL4474668)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)\C=C\C(=O)N1[C@H](Cc2ccccc2)C(OC)=CC1=O |r,c:52| Show InChI InChI=1S/C43H60N4O7/c1-10-30(6)40(46(7)8)43(52)54-37(24-29(4)5)42(51)45-34(23-28(2)3)41(50)44-33(25-31-17-13-11-14-18-31)21-22-38(48)47-35(36(53-9)27-39(47)49)26-32-19-15-12-16-20-32/h11-22,27-30,33-35,37,40H,10,23-26H2,1-9H3,(H,44,50)(H,45,51)/b22-21+/t30-,33+,34-,35+,37-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50505568

(CHEMBL4482954)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](CC(C)C)C(OC)=CC1=O |r,c:41| Show InChI InChI=1S/C34H58N4O7/c1-13-23(8)31(37(10)11)34(43)45-28(18-22(6)7)33(42)36-25(16-20(2)3)32(41)35-24(9)14-15-29(39)38-26(17-21(4)5)27(44-12)19-30(38)40/h14-15,19-26,28,31H,13,16-18H2,1-12H3,(H,35,41)(H,36,42)/b15-14+/t23-,24-,25-,26-,28-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50505567
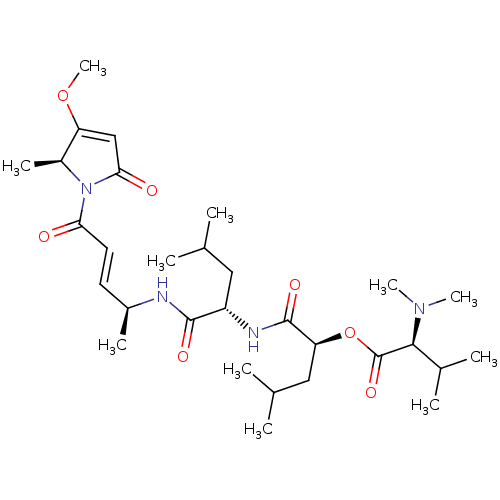
(CHEMBL4457500)Show SMILES COC1=CC(=O)N([C@H]1C)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)OC(=O)[C@H](C(C)C)N(C)C |r,t:2| Show InChI InChI=1S/C30H50N4O7/c1-17(2)14-22(32-29(38)24(15-18(3)4)41-30(39)27(19(5)6)33(9)10)28(37)31-20(7)12-13-25(35)34-21(8)23(40-11)16-26(34)36/h12-13,16-22,24,27H,14-15H2,1-11H3,(H,31,37)(H,32,38)/b13-12+/t20-,21-,22-,24-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... |
J Med Chem 62: 9026-9044 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00294
BindingDB Entry DOI: 10.7270/Q2H998GC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data