 Found 27682 hits with Last Name = 'li' and Initial = 'v'
Found 27682 hits with Last Name = 'li' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50398057
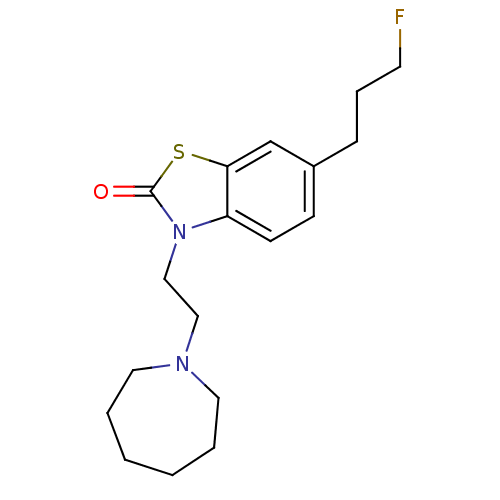
(CHEMBL2181924)Show InChI InChI=1S/C18H25FN2OS/c19-9-5-6-15-7-8-16-17(14-15)23-18(22)21(16)13-12-20-10-3-1-2-4-11-20/h7-8,14H,1-6,9-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50034407
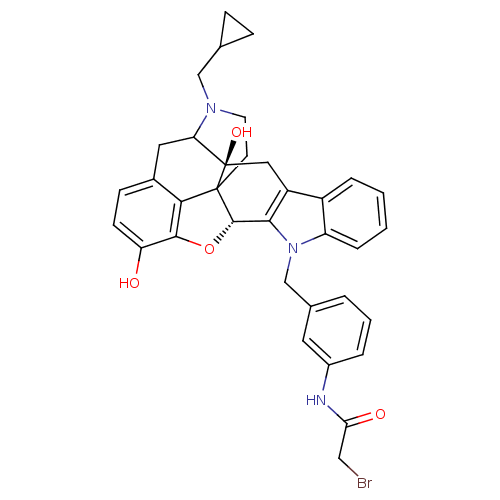
(1N-{3-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)c1c(C[C@@]35O)c2ccccc2n1Cc1cccc(NC(=O)CBr)c1 |TLB:23:22:18.4.5:7.13.12,THB:8:7:22:18.4.5,17:18:22:7.13.12| Show InChI InChI=1S/C35H34BrN3O4/c36-17-29(41)37-23-5-3-4-21(14-23)19-39-26-7-2-1-6-24(26)25-16-35(42)28-15-22-10-11-27(40)32-30(22)34(35,33(43-32)31(25)39)12-13-38(28)18-20-8-9-20/h1-7,10-11,14,20,28,33,40,42H,8-9,12-13,15-19H2,(H,37,41)/t28?,33-,34?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Tested for binding affinity against delta1 opioid receptor using [3H]-DPDPE as a radioligand in the guinea pig brain membranes. |
J Med Chem 38: 1337-43 (1995)
BindingDB Entry DOI: 10.7270/Q2416W3K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50060242
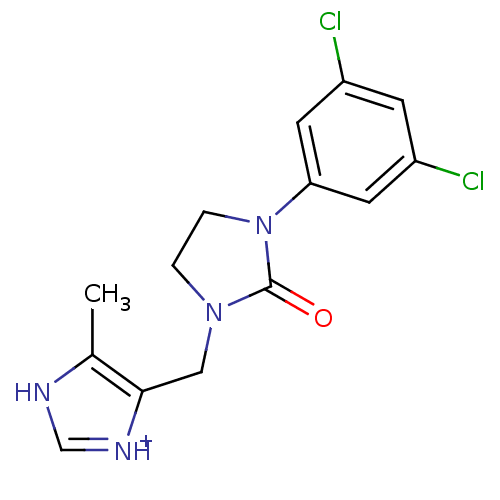
(5-[3-(3,5-Dichloro-phenyl)-2-oxo-imidazolidin-1-yl...)Show SMILES Cc1[nH]c[nH+]c1CN1CCN(C1=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C14H14Cl2N4O/c1-9-13(18-8-17-9)7-19-2-3-20(14(19)21)12-5-10(15)4-11(16)6-12/h4-6,8H,2-3,7H2,1H3,(H,17,18)/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia& Upjohn
Curated by ChEMBL
| Assay Description
In vitro affinity for 5-hydroxytryptamine 3 (5-HT3) receptor by displacement of [3H]BRL-43694 from rat entorhinal cortex |
J Med Chem 40: 3369-80 (1997)
Article DOI: 10.1021/jm970060o
BindingDB Entry DOI: 10.7270/Q2X63NNK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50034407

(1N-{3-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)c1c(C[C@@]35O)c2ccccc2n1Cc1cccc(NC(=O)CBr)c1 |TLB:23:22:18.4.5:7.13.12,THB:8:7:22:18.4.5,17:18:22:7.13.12| Show InChI InChI=1S/C35H34BrN3O4/c36-17-29(41)37-23-5-3-4-21(14-23)19-39-26-7-2-1-6-24(26)25-16-35(42)28-15-22-10-11-27(40)32-30(22)34(35,33(43-32)31(25)39)12-13-38(28)18-20-8-9-20/h1-7,10-11,14,20,28,33,40,42H,8-9,12-13,15-19H2,(H,37,41)/t28?,33-,34?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Tested for binding affinity against delta2 opioid receptor using [3H]-DSLET as a radioligand in the guinea pig brain membranes. |
J Med Chem 38: 1337-43 (1995)
BindingDB Entry DOI: 10.7270/Q2416W3K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549
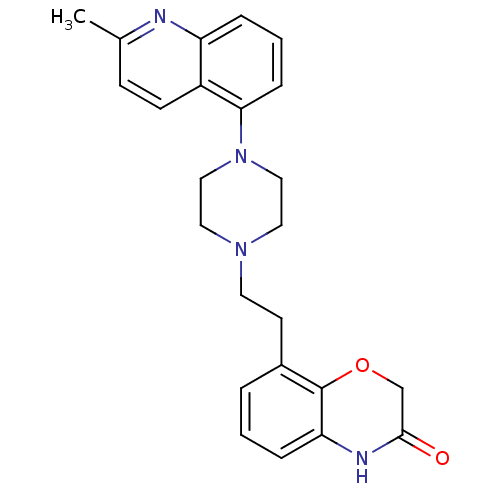
(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549

(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441
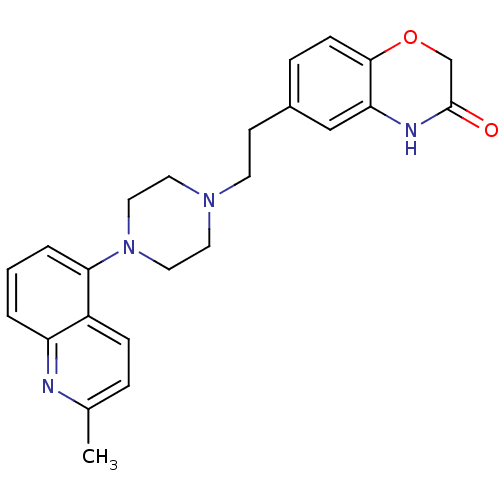
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413560
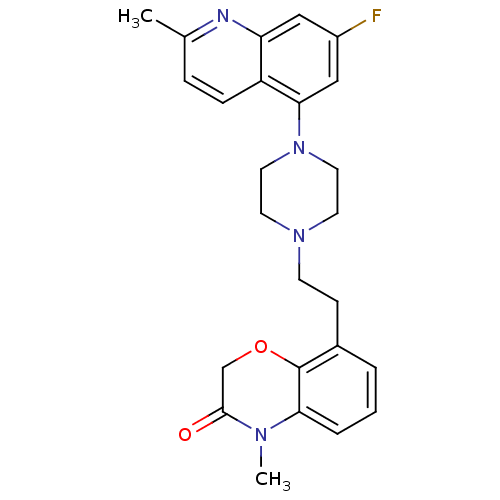
(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417409

(CHEMBL1290487)Show SMILES CN1C(=O)CCc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O/c1-19-9-10-22-23(27-19)6-4-8-25(22)30-17-15-29(16-18-30)14-13-20-5-3-7-24-21(20)11-12-26(31)28(24)2/h3-10H,11-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413077
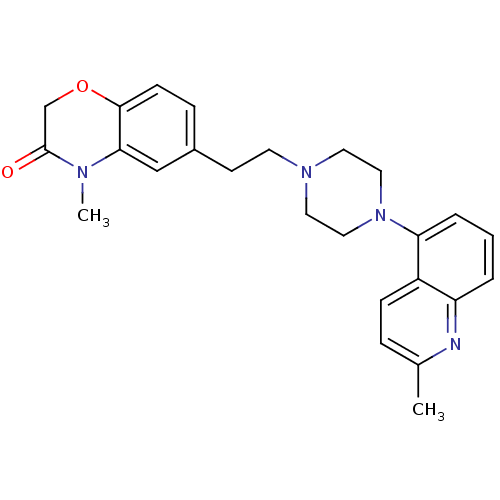
(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
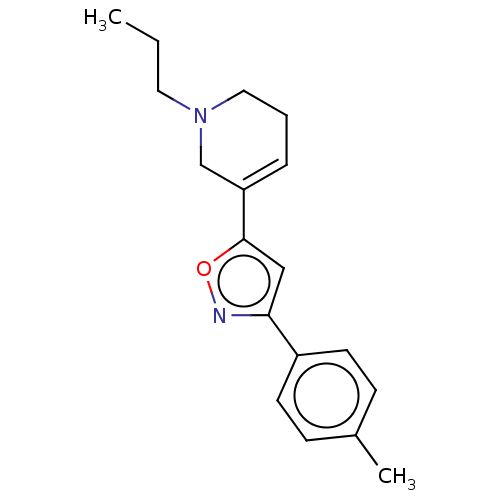
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120437
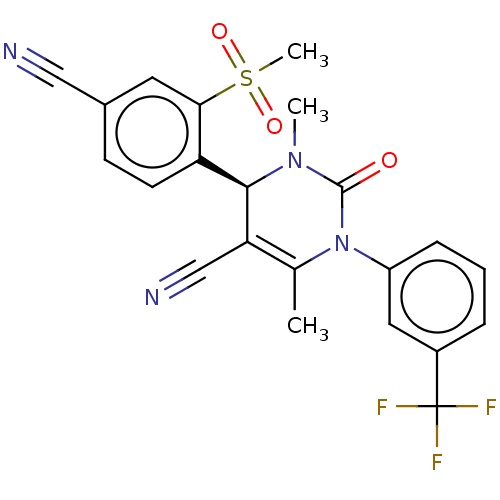
(CHEMBL3617973)Show SMILES CN1[C@@H](C(C#N)=C(C)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,t:5| Show InChI InChI=1S/C22H17F3N4O3S/c1-13-18(12-27)20(17-8-7-14(11-26)9-19(17)33(3,31)32)28(2)21(30)29(13)16-6-4-5-15(10-16)22(23,24)25/h4-10,20H,1-3H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413077

(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413555
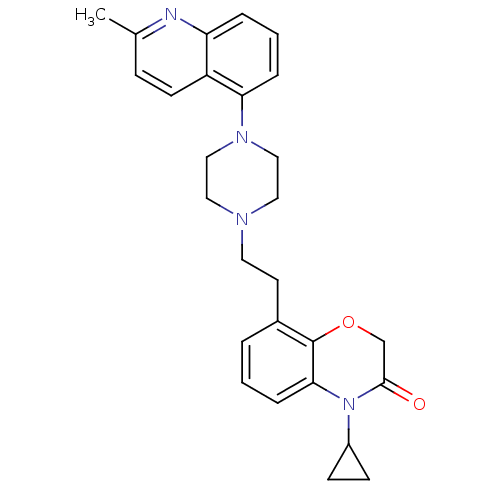
(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417420
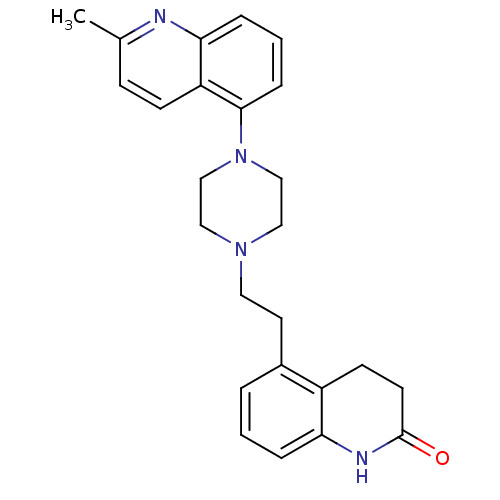
(CHEMBL1290486)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)CCc23)CC1 Show InChI InChI=1S/C25H28N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-9H,10-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413550
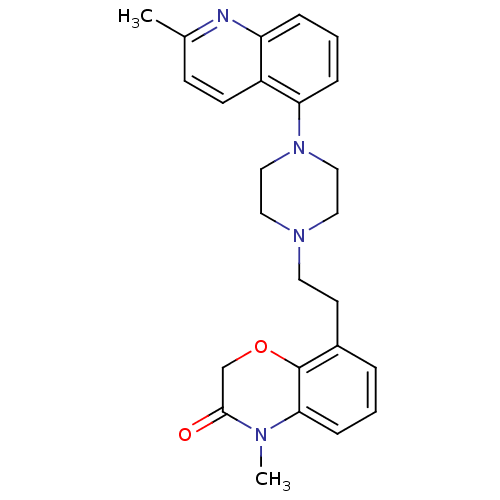
(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50326662
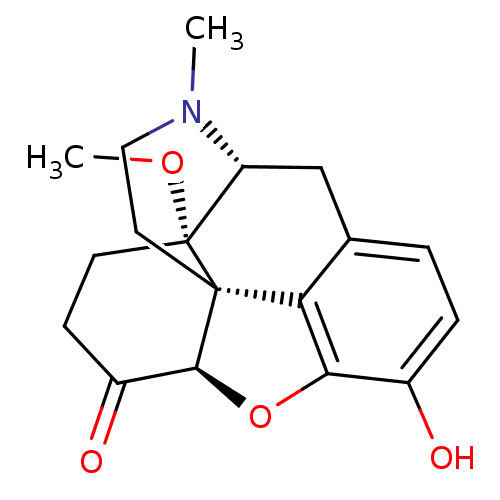
(10-hydroxy-17-methoxy-4-methyl-(13R,17S)-12-oxa-4-...)Show SMILES CO[C@]12CCC(=O)[C@@H]3Oc4c5c(C[C@H]1N(C)CC[C@@]235)ccc4O |r| Show InChI InChI=1S/C18H21NO4/c1-19-8-7-17-14-10-3-4-11(20)15(14)23-16(17)12(21)5-6-18(17,22-2)13(19)9-10/h3-4,13,16,20H,5-9H2,1-2H3/t13-,16+,17+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes incubated for 45 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01327
BindingDB Entry DOI: 10.7270/Q2RB7870 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50088398
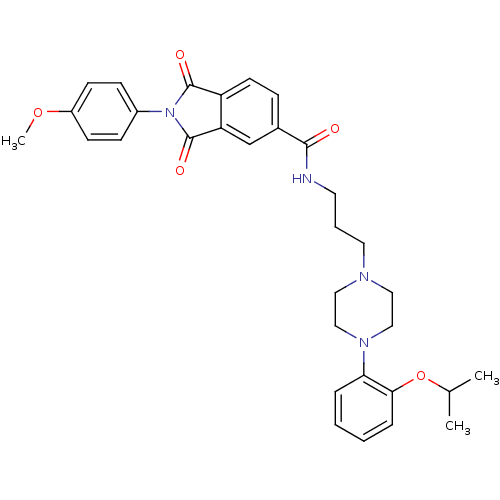
(2-(4-Methoxy-phenyl)-1,3-dioxo-2,3-dihydro-1H-isoi...)Show SMILES COc1ccc(cc1)N1C(=O)c2ccc(cc2C1=O)C(=O)NCCCN1CCN(CC1)c1ccccc1OC(C)C Show InChI InChI=1S/C32H36N4O5/c1-22(2)41-29-8-5-4-7-28(29)35-19-17-34(18-20-35)16-6-15-33-30(37)23-9-14-26-27(21-23)32(39)36(31(26)38)24-10-12-25(40-3)13-11-24/h4-5,7-14,21-22H,6,15-20H2,1-3H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human alpha-1A adrenergic receptor was determined using [125]-HEAT as radioligand |
J Med Chem 43: 2183-95 (2000)
BindingDB Entry DOI: 10.7270/Q2XW4J20 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
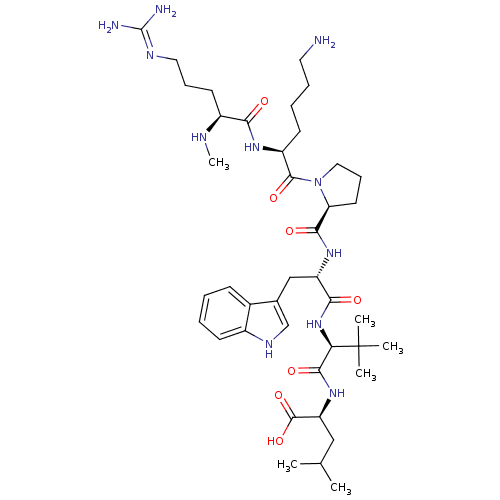
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50041293
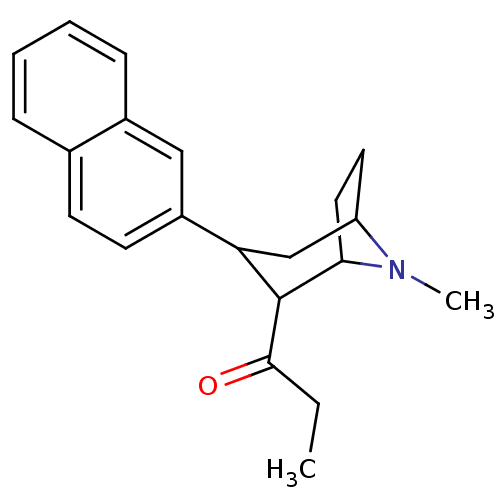
(1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...)Show SMILES CCC(=O)C1C2CCC(CC1c1ccc3ccccc3c1)N2C |THB:22:21:4.10.9:6.7,11:10:21:6.7,2:4:21:6.7| Show InChI InChI=1S/C21H25NO/c1-3-20(23)21-18(13-17-10-11-19(21)22(17)2)16-9-8-14-6-4-5-7-15(14)12-16/h4-9,12,17-19,21H,3,10-11,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. |
J Med Chem 37: 1262-8 (1994)
BindingDB Entry DOI: 10.7270/Q2TH8NBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417424
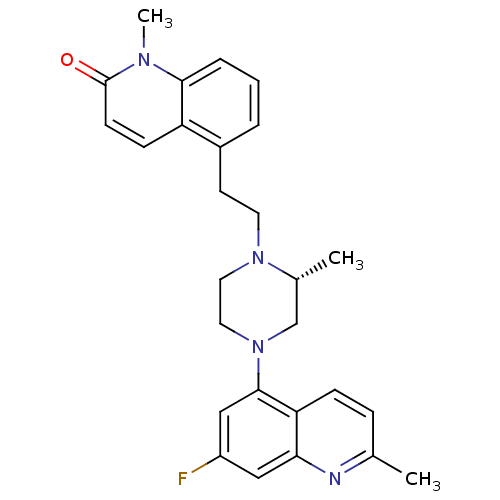
(CHEMBL1289394)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2n(C)c(=O)ccc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C27H29FN4O/c1-18-7-8-23-24(29-18)15-21(28)16-26(23)32-14-13-31(19(2)17-32)12-11-20-5-4-6-25-22(20)9-10-27(33)30(25)3/h4-10,15-16,19H,11-14,17H2,1-3H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417411
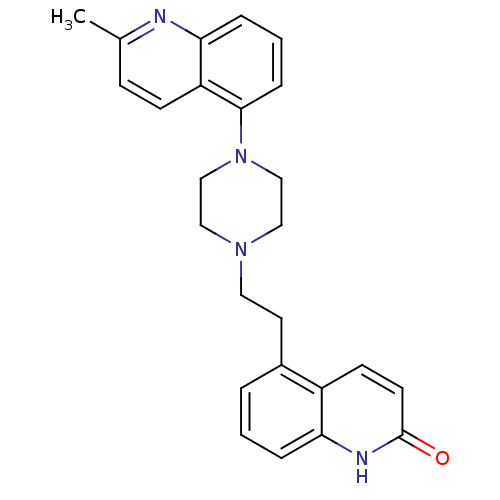
(CHEMBL1290715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3[nH]c(=O)ccc23)CC1 Show InChI InChI=1S/C25H26N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-11H,12-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413086
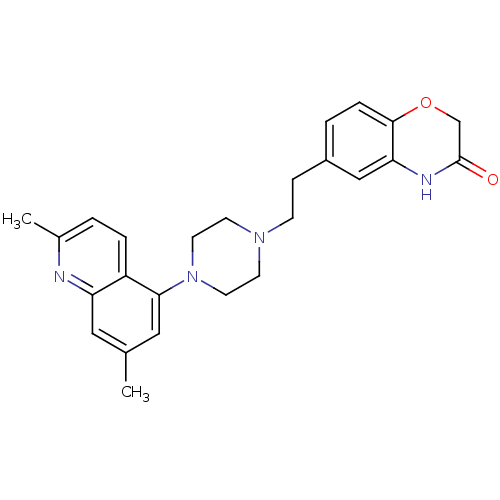
(CHEMBL484260)Show SMILES Cc1cc(N2CCN(CCc3ccc4OCC(=O)Nc4c3)CC2)c2ccc(C)nc2c1 Show InChI InChI=1S/C25H28N4O2/c1-17-13-21-20(5-3-18(2)26-21)23(14-17)29-11-9-28(10-12-29)8-7-19-4-6-24-22(15-19)27-25(30)16-31-24/h3-6,13-15H,7-12,16H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413553
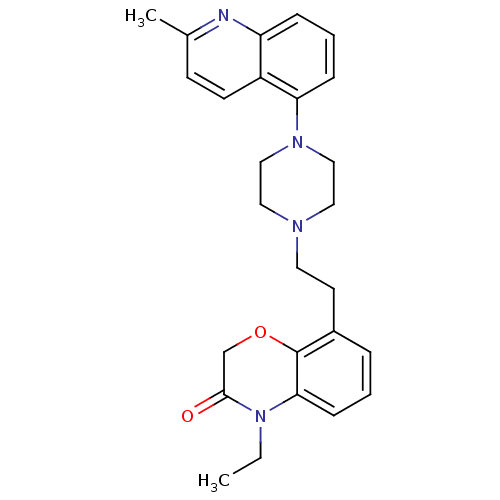
(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964
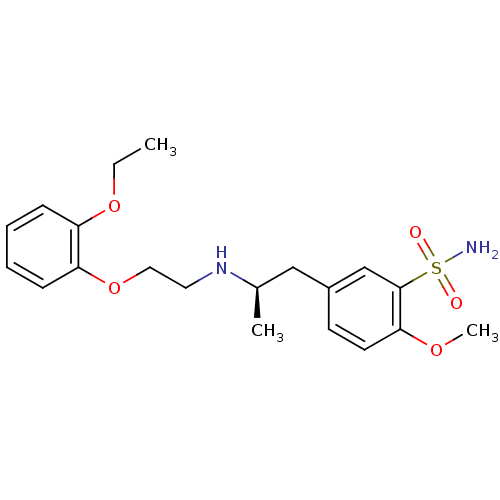
((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human alpha-1A adrenergic receptor was determined using [125]-HEAT as radioligand |
J Med Chem 43: 2183-95 (2000)
BindingDB Entry DOI: 10.7270/Q2XW4J20 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211341

(5-bromo-6-chloro-N-((1s,4s)-4-(4-(2-isopropoxyphen...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)c1cnc(Cl)c(Br)c1 |wU:16.17,19.24,(-7.75,-8.21,;-8.54,-9.53,;-10.08,-9.51,;-7.79,-10.88,;-8.57,-12.2,;-10.11,-12.18,;-10.9,-13.51,;-10.14,-14.86,;-8.6,-14.87,;-7.82,-13.55,;-6.29,-13.56,;-5.53,-14.9,;-3.99,-14.92,;-3.21,-13.6,;-3.95,-12.26,;-5.49,-12.24,;-1.67,-13.62,;-.92,-14.97,;.61,-14.99,;1.41,-13.67,;.65,-12.32,;-.89,-12.3,;2.96,-13.7,;3.74,-12.37,;2.41,-11.59,;5.06,-13.14,;4.5,-11.03,;6.05,-11.02,;6.8,-9.68,;6.02,-8.35,;6.78,-7.01,;4.47,-8.37,;3.69,-7.05,;3.72,-9.71,)| Show InChI InChI=1S/C24H32BrClN4O3S/c1-17(2)33-23-6-4-3-5-22(23)30-13-11-29(12-14-30)19-9-7-18(8-10-19)28-34(31,32)20-15-21(25)24(26)27-16-20/h3-6,15-19,28H,7-14H2,1-2H3/t18-,19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50454871
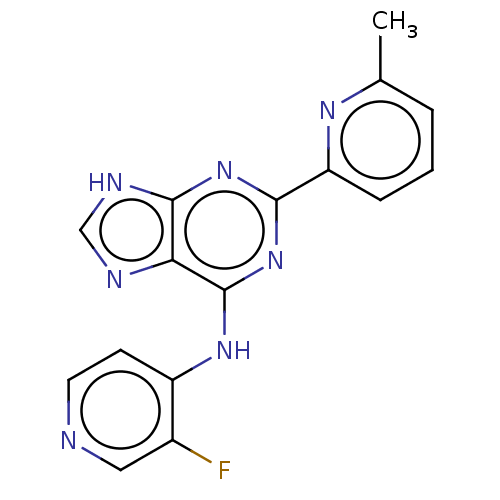
(CHEMBL4209835)Show InChI InChI=1S/C16H12FN7/c1-9-3-2-4-12(21-9)14-23-15-13(19-8-20-15)16(24-14)22-11-5-6-18-7-10(11)17/h2-8H,1H3,(H2,18,19,20,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412734

(N-(3-fluoropyridin-4-yl)-2-(6-methylpyridin-2-yl)-...)Show InChI InChI=1S/C17H13FN6/c1-10-3-2-4-14(21-10)17-23-15-11(5-8-20-15)16(24-17)22-13-6-7-19-9-12(13)18/h2-9H,1H3,(H2,19,20,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM412755
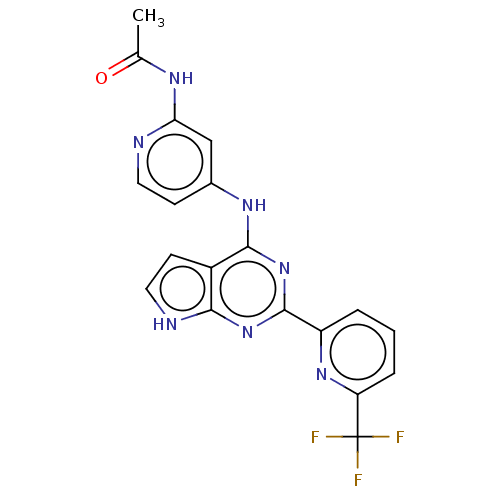
(N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...)Show SMILES CC(=O)Nc1cc(Nc2nc(nc3[nH]ccc23)-c2cccc(n2)C(F)(F)F)ccn1 Show InChI InChI=1S/C19H14F3N7O/c1-10(30)25-15-9-11(5-7-23-15)26-17-12-6-8-24-16(12)28-18(29-17)13-3-2-4-14(27-13)19(20,21)22/h2-9H,1H3,(H3,23,24,25,26,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay |
Bioorg Med Chem 26: 1026-1034 (2018)
Article DOI: 10.1016/j.bmc.2018.01.014
BindingDB Entry DOI: 10.7270/Q27M0BHV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413078
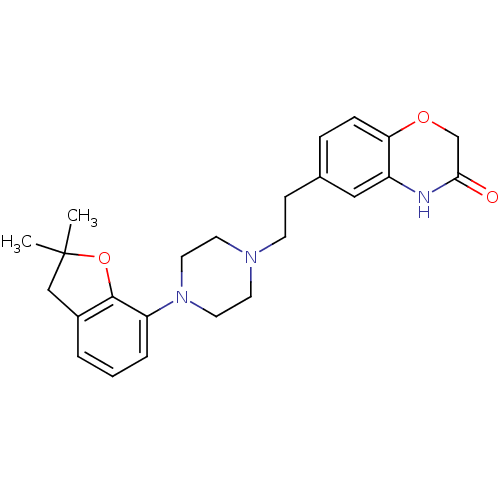
(CHEMBL491839)Show SMILES CC1(C)Cc2cccc(N3CCN(CCc4ccc5OCC(=O)Nc5c4)CC3)c2O1 Show InChI InChI=1S/C24H29N3O3/c1-24(2)15-18-4-3-5-20(23(18)30-24)27-12-10-26(11-13-27)9-8-17-6-7-21-19(14-17)25-22(28)16-29-21/h3-7,14H,8-13,15-16H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413084
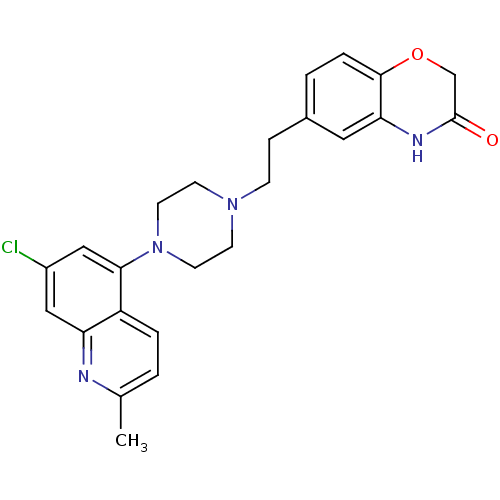
(CHEMBL521506)Show SMILES Cc1ccc2c(cc(Cl)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50413083
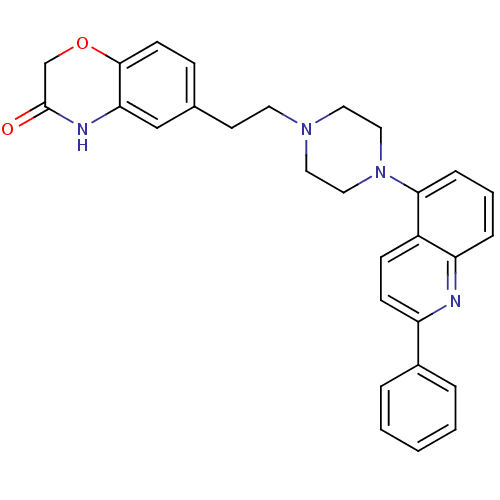
(CHEMBL484059)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4nc(ccc34)-c3ccccc3)cc2N1 Show InChI InChI=1S/C29H28N4O2/c34-29-20-35-28-12-9-21(19-26(28)31-29)13-14-32-15-17-33(18-16-32)27-8-4-7-25-23(27)10-11-24(30-25)22-5-2-1-3-6-22/h1-12,19H,13-18,20H2,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human SerT expressed pig LLCPK cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413555

(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50201397
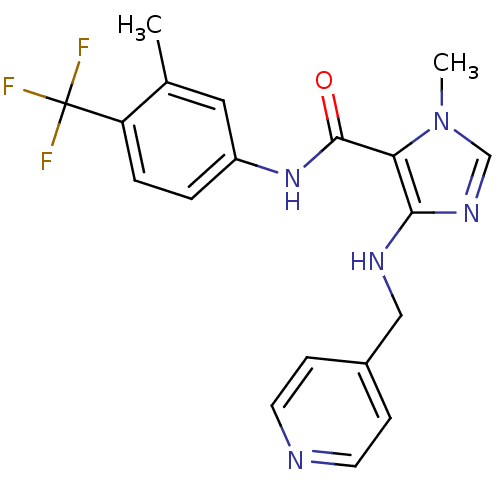
(1-methyl-N-(3-methyl-4-(trifluoromethyl)phenyl)-4-...)Show SMILES Cc1cc(NC(=O)c2c(NCc3ccncc3)ncn2C)ccc1C(F)(F)F Show InChI InChI=1S/C19H18F3N5O/c1-12-9-14(3-4-15(12)19(20,21)22)26-18(28)16-17(25-11-27(16)2)24-10-13-5-7-23-8-6-13/h3-9,11,24H,10H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 by [32P]ATP-competitive radioassay |
Bioorg Med Chem Lett 17: 1369-75 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.087
BindingDB Entry DOI: 10.7270/Q2125S92 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50088405
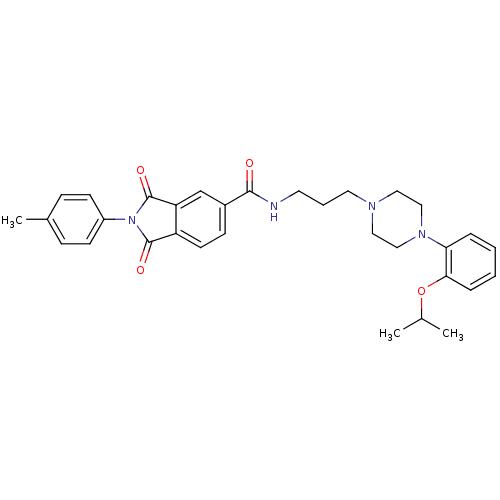
(1,3-Dioxo-2-p-tolyl-2,3-dihydro-1H-isoindole-5-car...)Show SMILES CC(C)Oc1ccccc1N1CCN(CCCNC(=O)c2ccc3C(=O)N(C(=O)c3c2)c2ccc(C)cc2)CC1 Show InChI InChI=1S/C32H36N4O4/c1-22(2)40-29-8-5-4-7-28(29)35-19-17-34(18-20-35)16-6-15-33-30(37)24-11-14-26-27(21-24)32(39)36(31(26)38)25-12-9-23(3)10-13-25/h4-5,7-14,21-22H,6,15-20H2,1-3H3,(H,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human alpha-1A adrenergic receptor was determined using [125]-HEAT as radioligand |
J Med Chem 43: 2183-95 (2000)
BindingDB Entry DOI: 10.7270/Q2XW4J20 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118028
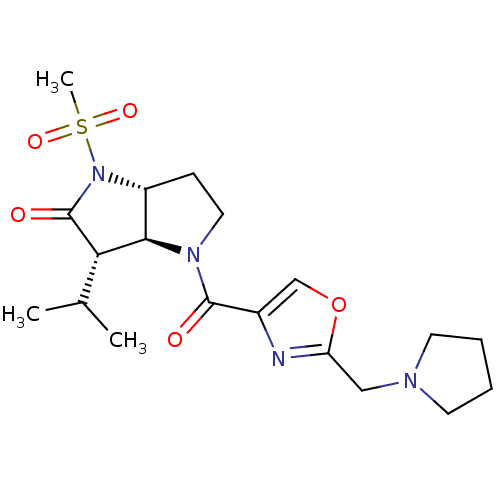
(3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2coc(CN3CCCC3)n2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H28N4O5S/c1-12(2)16-17-14(23(19(16)25)29(3,26)27)6-9-22(17)18(24)13-11-28-15(20-13)10-21-7-4-5-8-21/h11-12,14,16-17H,4-10H2,1-3H3/t14-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15131
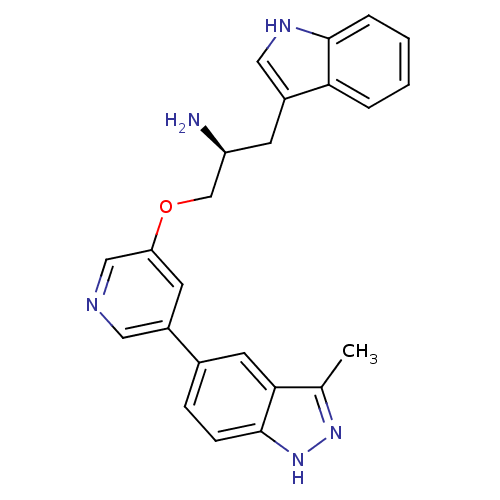
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019
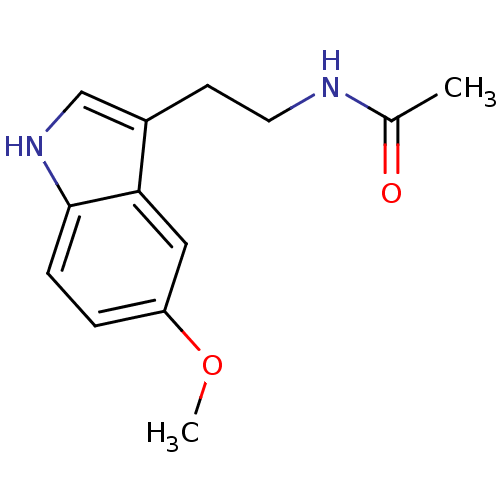
(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma
Curated by ChEMBL
| Assay Description
Binding Affinity (pKi) towards human Melatonin receptor type 1A |
J Med Chem 46: 1429-39 (2003)
Article DOI: 10.1021/jm020982d
BindingDB Entry DOI: 10.7270/Q2DN47T2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50201363
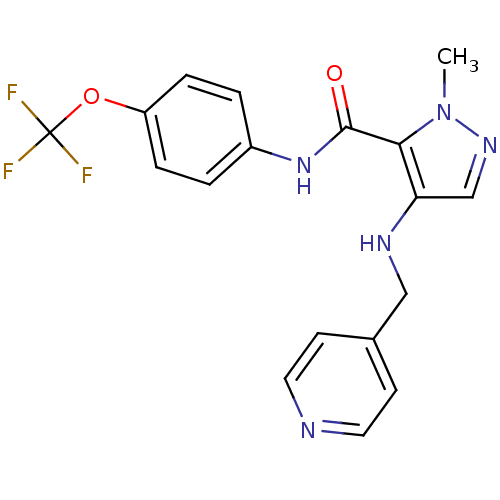
(1-methyl-4-(pyridin-4-ylmethylamino)-N-(4-(trifluo...)Show SMILES Cn1ncc(NCc2ccncc2)c1C(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C18H16F3N5O2/c1-26-16(15(11-24-26)23-10-12-6-8-22-9-7-12)17(27)25-13-2-4-14(5-3-13)28-18(19,20)21/h2-9,11,23H,10H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 by [32P]ATP-competitive radioassay |
Bioorg Med Chem Lett 17: 1369-75 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.087
BindingDB Entry DOI: 10.7270/Q2125S92 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50088389
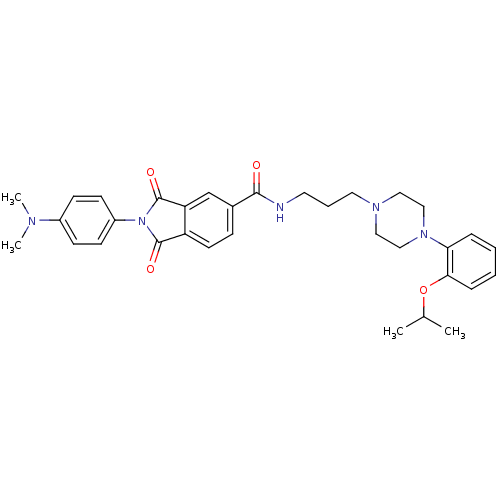
(2-(4-Dimethylamino-phenyl)-1,3-dioxo-2,3-dihydro-1...)Show SMILES CC(C)Oc1ccccc1N1CCN(CCCNC(=O)c2ccc3C(=O)N(C(=O)c3c2)c2ccc(cc2)N(C)C)CC1 Show InChI InChI=1S/C33H39N5O4/c1-23(2)42-30-9-6-5-8-29(30)37-20-18-36(19-21-37)17-7-16-34-31(39)24-10-15-27-28(22-24)33(41)38(32(27)40)26-13-11-25(12-14-26)35(3)4/h5-6,8-15,22-23H,7,16-21H2,1-4H3,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human alpha-1A adrenergic receptor was determined using [125]-HEAT as radioligand |
J Med Chem 43: 2183-95 (2000)
BindingDB Entry DOI: 10.7270/Q2XW4J20 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50318267
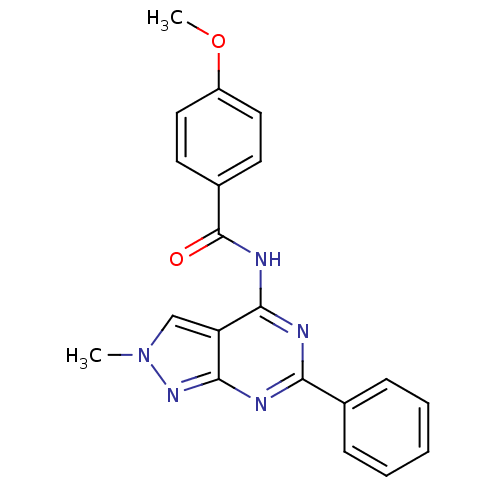
(4-methoxy-N-(2-methyl-6-phenyl-2H-pyrazolo[3,4-d]p...)Show SMILES COc1ccc(cc1)C(=O)Nc1nc(nc2nn(C)cc12)-c1ccccc1 Show InChI InChI=1S/C20H17N5O2/c1-25-12-16-18(23-20(26)14-8-10-15(27-2)11-9-14)21-17(22-19(16)24-25)13-6-4-3-5-7-13/h3-12H,1-2H3,(H,21,22,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 53: 3954-63 (2010)
Article DOI: 10.1021/jm901785w
BindingDB Entry DOI: 10.7270/Q2N017HH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human alpha-1D adrenergic receptor was determined using [125]-HEAT as radioligand |
J Med Chem 43: 2183-95 (2000)
BindingDB Entry DOI: 10.7270/Q2XW4J20 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM86846
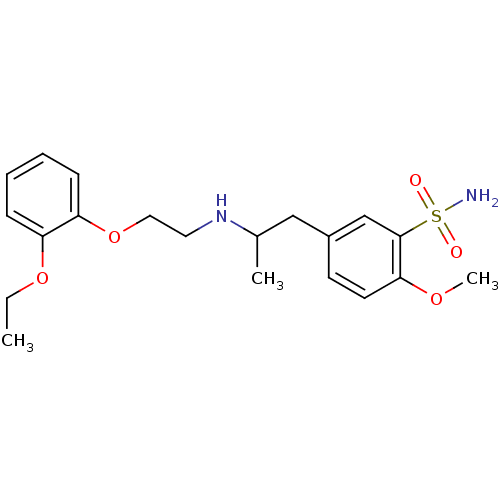
(CAS_106133-20-4 | NSC_60147 | Tamsulosin)Show SMILES CCOc1ccccc1OCCNC(C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50548314
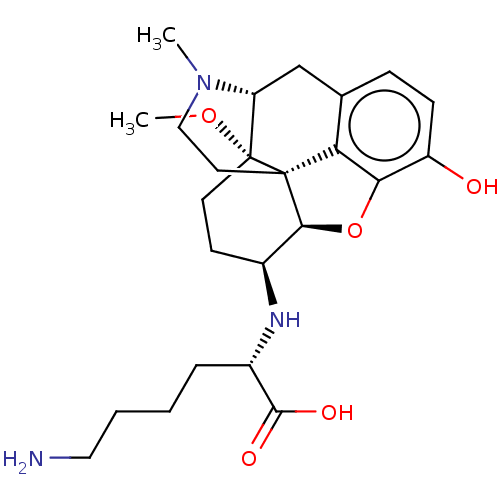
(CHEMBL4793888)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@]5(CC[C@@H]2N[C@@H](CCCCN)C(O)=O)OC)ccc3O |r,THB:10:9:14:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes incubated for 45 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01327
BindingDB Entry DOI: 10.7270/Q2RB7870 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM86846

(CAS_106133-20-4 | NSC_60147 | Tamsulosin)Show SMILES CCOc1ccccc1OCCNC(C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data