

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM14338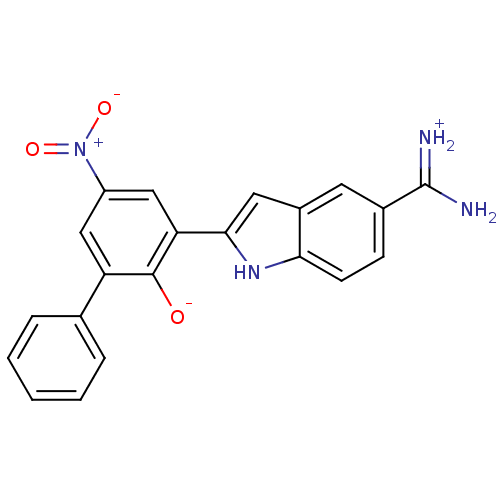 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14352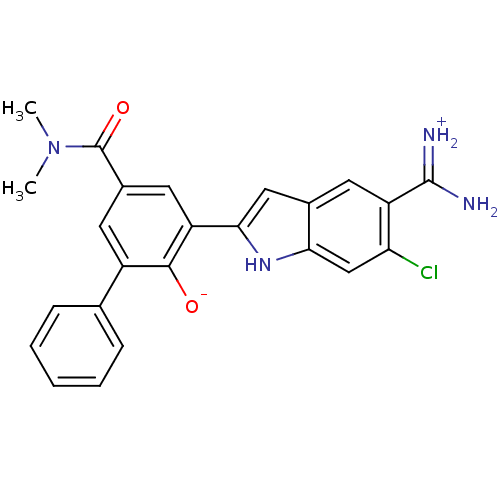 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14351 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14354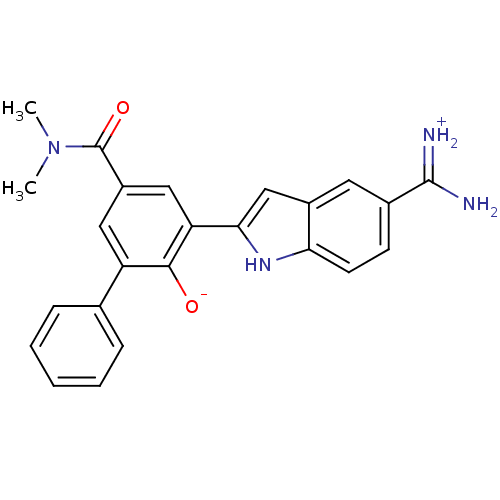 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50187800 ((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to human tryptase beta2 | Bioorg Med Chem Lett 16: 4085-9 (2006) Article DOI: 10.1016/j.bmcl.2006.04.088 BindingDB Entry DOI: 10.7270/Q2X929W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase (Rattus norvegicus) | BDBM50187800 ((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to rat tryptase | Bioorg Med Chem Lett 16: 4085-9 (2006) Article DOI: 10.1016/j.bmcl.2006.04.088 BindingDB Entry DOI: 10.7270/Q2X929W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14142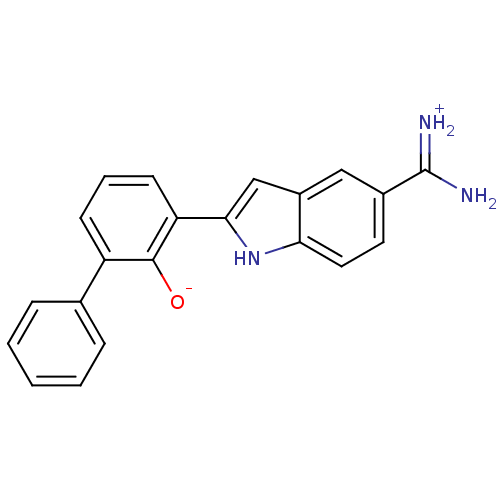 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.45 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14152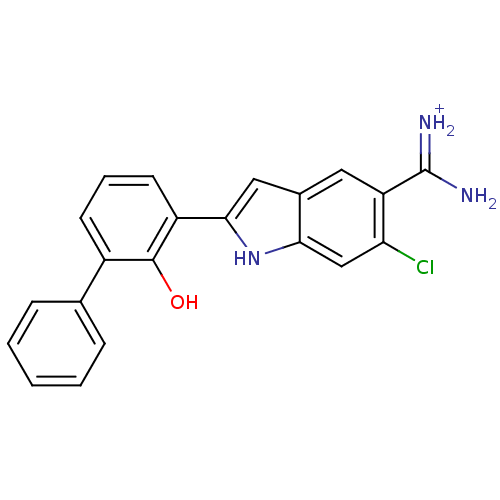 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14335 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-chl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14337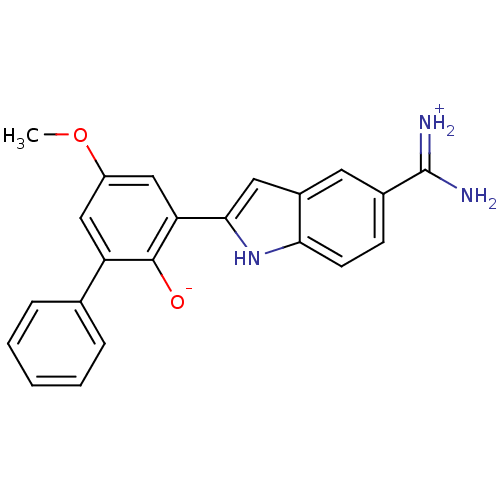 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14329 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14150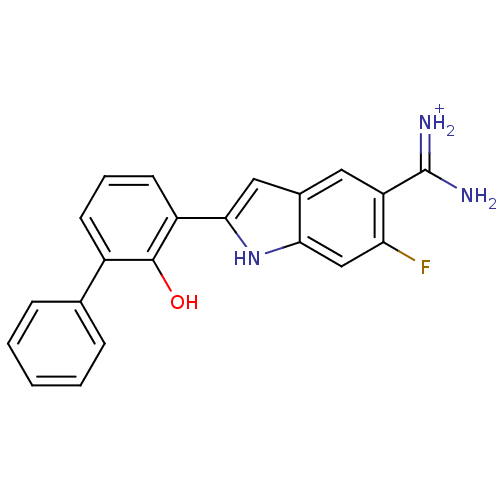 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14334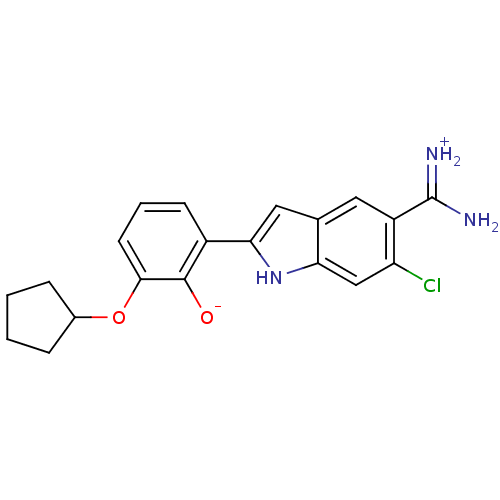 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14353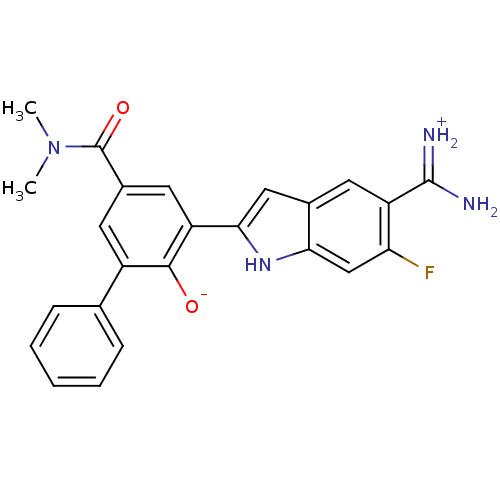 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14344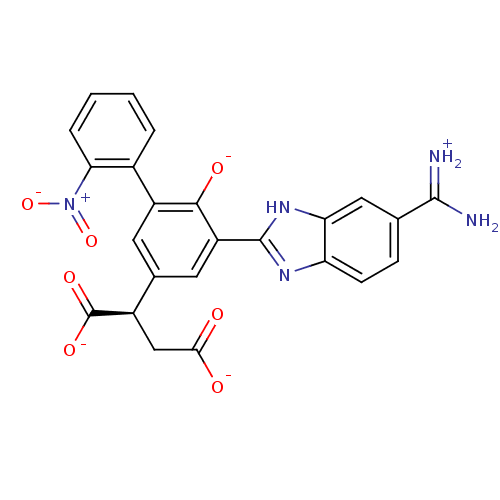 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-1,3-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14337 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14350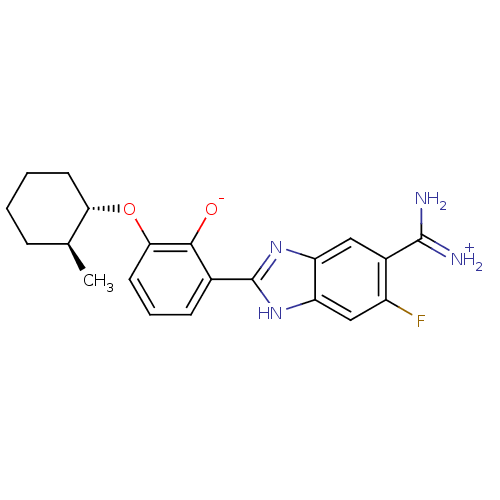 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM14354 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14330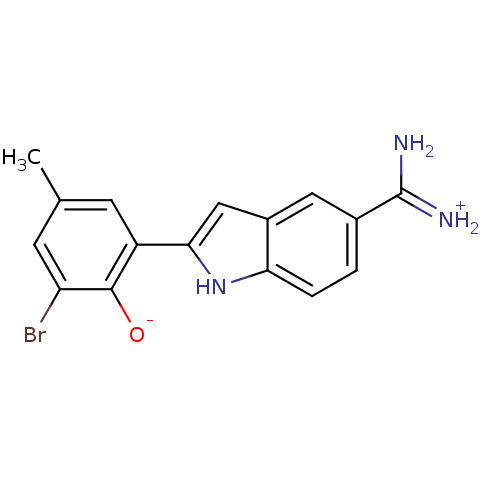 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14349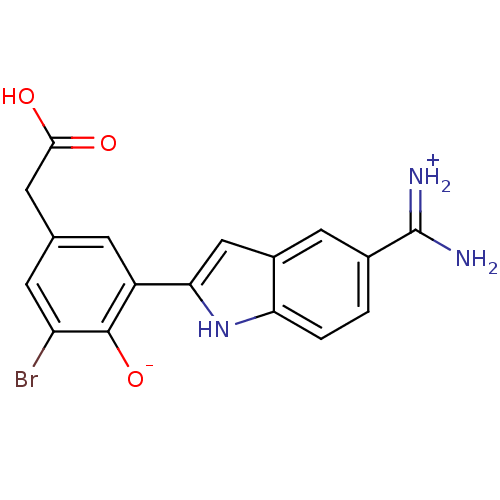 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase (Mus musculus) | BDBM50187800 ((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to mouse tryptase | Bioorg Med Chem Lett 16: 4085-9 (2006) Article DOI: 10.1016/j.bmcl.2006.04.088 BindingDB Entry DOI: 10.7270/Q2X929W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Tryptase beta-2 (Homo sapiens (Human)) | BDBM50187800 ((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to recombinant cynomolgus monkey tryptase | Bioorg Med Chem Lett 16: 4085-9 (2006) Article DOI: 10.1016/j.bmcl.2006.04.088 BindingDB Entry DOI: 10.7270/Q2X929W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14346 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-indol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14349 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14330 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 50 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.02 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14354 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14144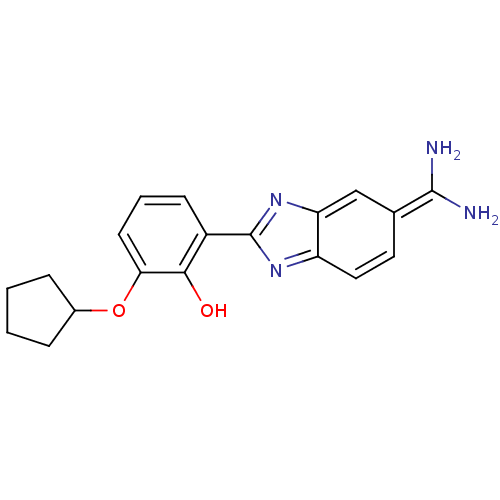 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.67 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14330 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14348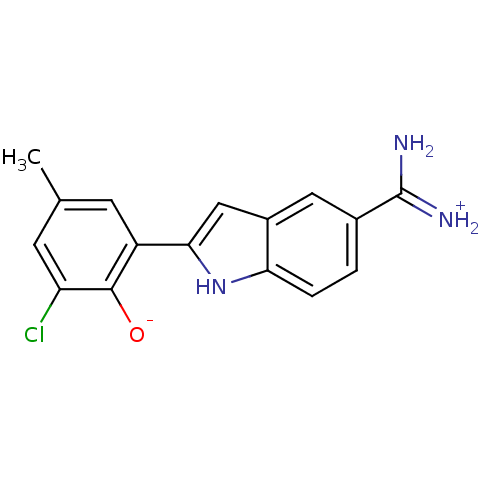 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14332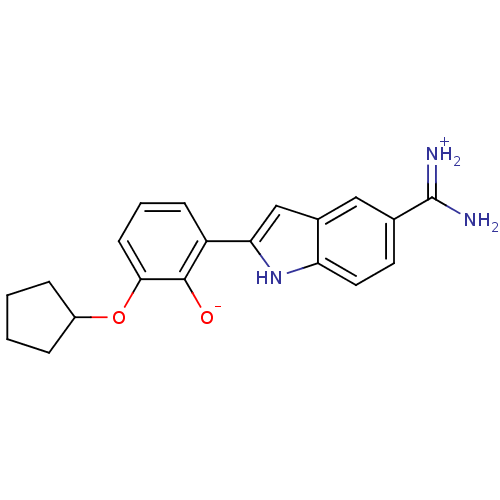 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-(cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 68 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.06 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14142 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14325 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 74 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14342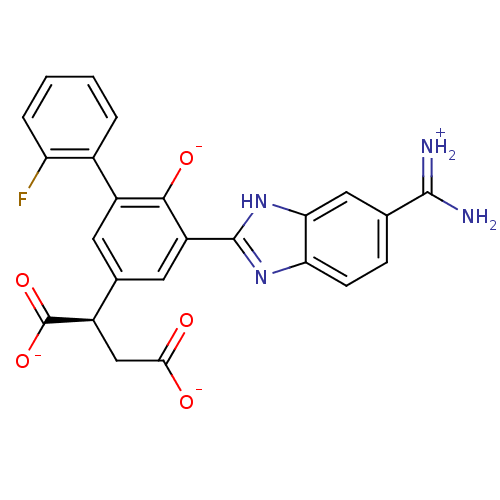 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-1,3-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14348 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14152 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.72 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14356 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-indol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14358 (4-(2-aminiumylethyl)-2-{5-[amino(iminiumyl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14148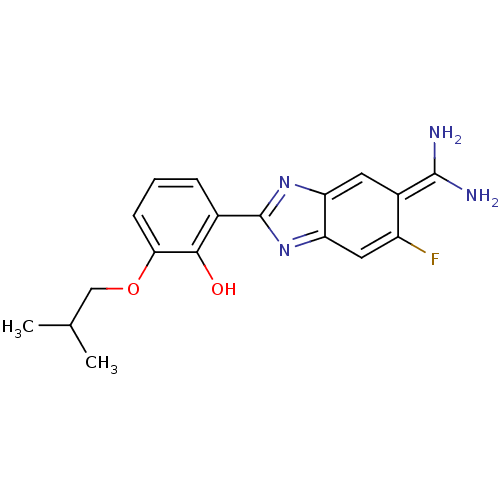 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14340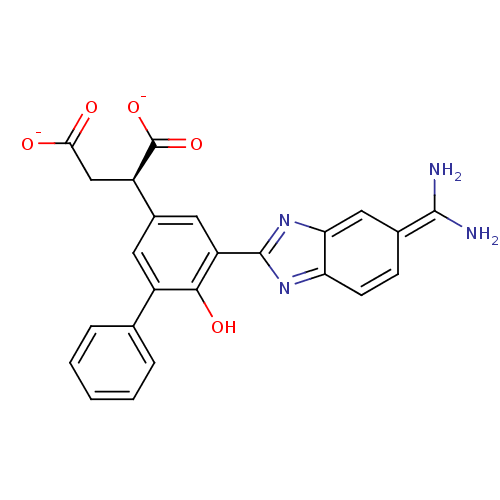 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-1,3-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14145 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.45 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM14353 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM14337 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14330 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-bro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14337 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14334 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14142 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 404 total ) | Next | Last >> |