 Found 1113 hits with Last Name = 'fan' and Initial = 'w'
Found 1113 hits with Last Name = 'fan' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
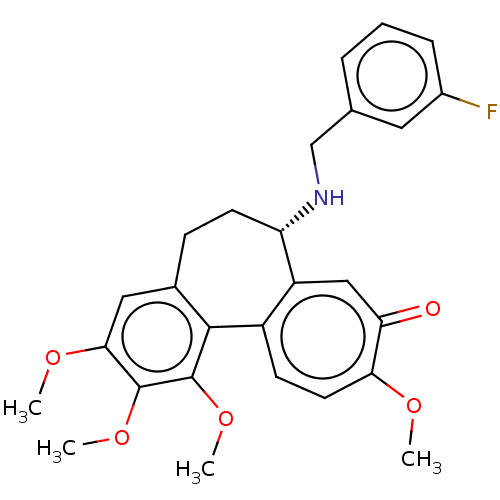
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
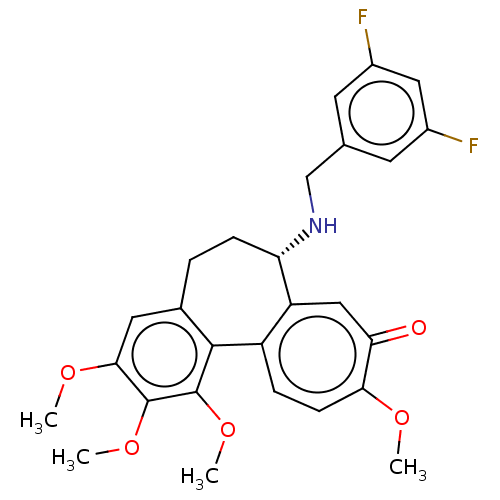
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86434
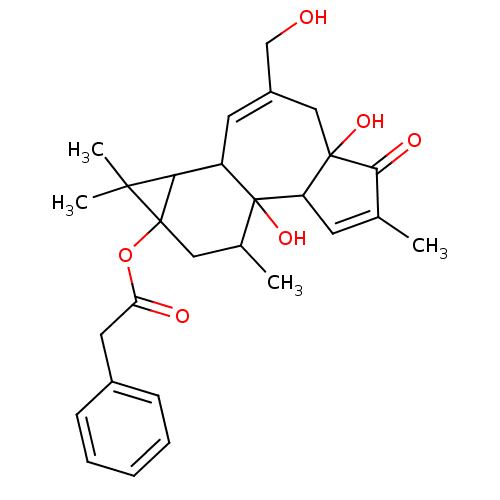
(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
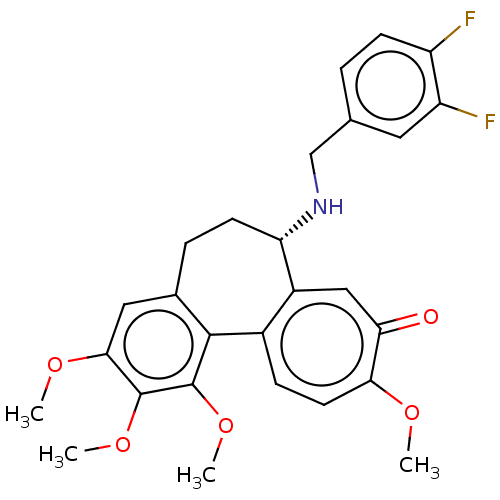
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
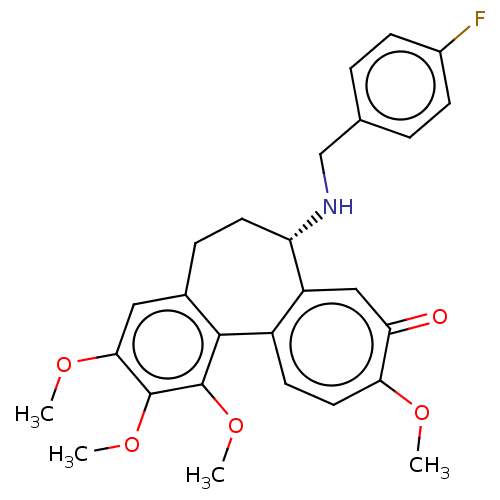
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86429
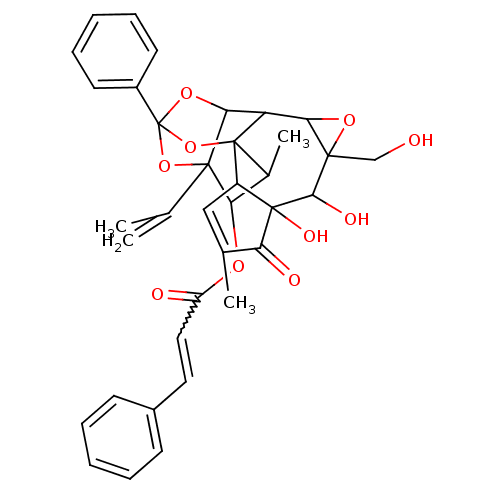
(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86431
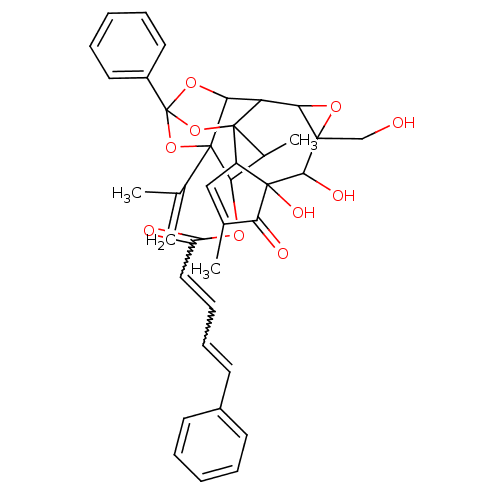
(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
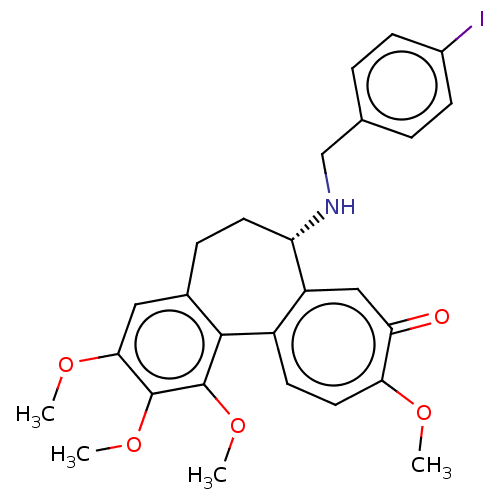
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50537515
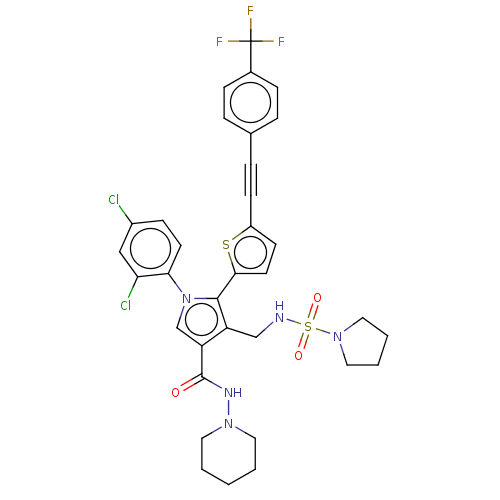
(CHEMBL4644088)Show SMILES FC(F)(F)c1ccc(cc1)C#Cc1ccc(s1)-c1c(CNS(=O)(=O)N2CCCC2)c(cn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C34H32Cl2F3N5O3S2/c35-25-11-14-30(29(36)20-25)44-22-28(33(45)41-42-16-2-1-3-17-42)27(21-40-49(46,47)43-18-4-5-19-43)32(44)31-15-13-26(48-31)12-8-23-6-9-24(10-7-23)34(37,38)39/h6-7,9-11,13-15,20,22,40H,1-5,16-19,21H2,(H,41,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha expressed in Sf-9 cells |
Eur J Med Chem 162: 679-734 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.017
BindingDB Entry DOI: 10.7270/Q21839DX |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86434

(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485940

(CHEMBL2181005)Show SMILES COc1cc2CC[C@H](NCc3ccc(Br)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28BrNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM239073

(US9416103, CP-55,940)Show SMILES CCCCCCC(C)(C)c1ccc(C2CC(O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20?,22?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas
US Patent
| Assay Description
Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... |
US Patent US9416103 (2016)
BindingDB Entry DOI: 10.7270/Q2HX1BKQ |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86431

(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86432
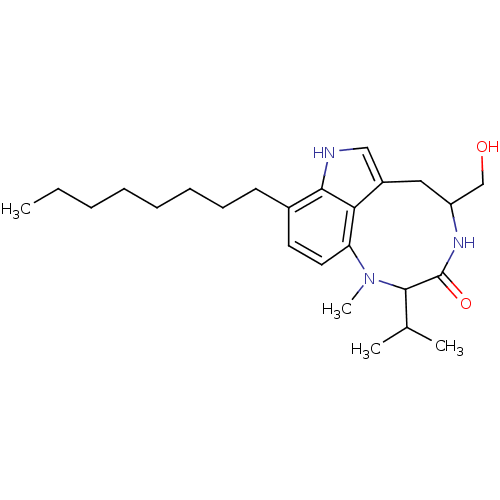
((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86432

((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86431

(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86434

(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]Diprenorphine from rat kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50036649
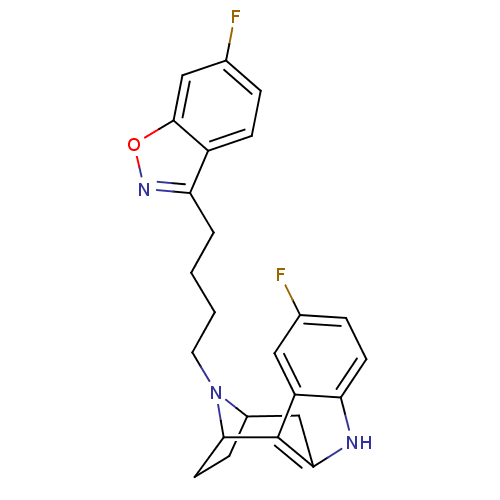
((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...)Show SMILES Fc1ccc2c(CCCCN3C4CCC3c3c(C4)[nH]c4ccc(F)cc34)noc2c1 |TLB:9:10:15.16.17:13.12,THB:25:15:10:13.12,18:16:10:13.12| Show InChI InChI=1S/C24H23F2N3O/c25-14-5-8-19-18(11-14)24-21(27-19)13-16-6-9-22(24)29(16)10-2-1-3-20-17-7-4-15(26)12-23(17)30-28-20/h4-5,7-8,11-12,16,22,27H,1-3,6,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50036649

((7R,10S)-1-(6-fluorobenzo[d]isoxazol-3-yl)-4-[5-fl...)Show SMILES Fc1ccc2c(CCCCN3C4CCC3c3c(C4)[nH]c4ccc(F)cc34)noc2c1 |TLB:9:10:15.16.17:13.12,THB:25:15:10:13.12,18:16:10:13.12| Show InChI InChI=1S/C24H23F2N3O/c25-14-5-8-19-18(11-14)24-21(27-19)13-16-6-9-22(24)29(16)10-2-1-3-20-17-7-4-15(26)12-23(17)30-28-20/h4-5,7-8,11-12,16,22,27H,1-3,6,9-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86432

((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50036648
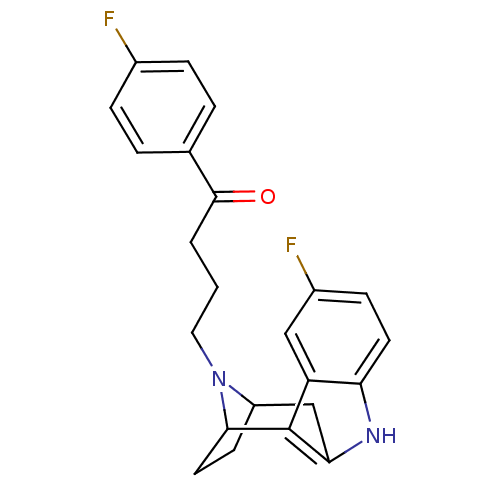
((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1c1c(C2)[nH]c2ccc(F)cc12 |TLB:11:12:17.18.19:15.14,THB:27:17:12:15.14,20:18:12:15.14| Show InChI InChI=1S/C23H22F2N2O/c24-15-5-3-14(4-6-15)22(28)2-1-11-27-17-8-10-21(27)23-18-12-16(25)7-9-19(18)26-20(23)13-17/h3-7,9,12,17,21,26H,1-2,8,10-11,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards 5-hydroxytryptamine 2 receptor in rat striatal membranes by [3H]ketanserin displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM239078

(US9416103, TV-5-157)Show InChI InChI=1S/C24H23NO2/c1-3-4-15-25-16-21(19-12-8-14-22(27-2)23(19)25)24(26)20-13-7-10-17-9-5-6-11-18(17)20/h5-14,16H,3-4,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... |
J Med Chem 56: 4537-50 (2013)
Article DOI: 10.1021/jm400268b
BindingDB Entry DOI: 10.7270/Q25M68M1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM239078

(US9416103, TV-5-157)Show InChI InChI=1S/C24H23NO2/c1-3-4-15-25-16-21(19-12-8-14-22(27-2)23(19)25)24(26)20-13-7-10-17-9-5-6-11-18(17)20/h5-14,16H,3-4,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas
US Patent
| Assay Description
A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... |
US Patent US9416103 (2016)
BindingDB Entry DOI: 10.7270/Q2HX1BKQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Mus musculus (Mouse)) | BDBM50046475

(4-[9,15-diazatetracyclo[10.2.1.02,10.03,8]pentadec...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1c1c(C2)[nH]c2ccccc12 |TLB:11:12:17.18.19:15.14,THB:20:18:12:15.14,26:17:12:15.14| Show InChI InChI=1S/C23H23FN2O/c24-16-9-7-15(8-10-16)22(27)6-3-13-26-17-11-12-21(26)23-18-4-1-2-5-19(18)25-20(23)14-17/h1-2,4-5,7-10,17,21,25H,3,6,11-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Mus musculus (Mouse)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50297345
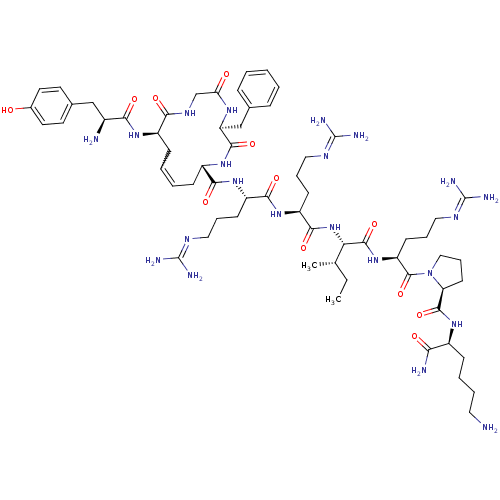
((5S,8S,13R,Z)-13-((S)-2-amino-3-(4-hydroxyphenyl)p...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]=[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r,w:33.33| Show InChI InChI=1S/C63H100N22O12/c1-3-36(2)50(59(96)83-46(22-13-31-75-63(71)72)60(97)85-32-14-23-48(85)58(95)78-41(51(66)88)17-9-10-28-64)84-56(93)45(21-12-30-74-62(69)70)81-55(92)44(20-11-29-73-61(67)68)80-54(91)43-19-8-7-18-42(79-52(89)40(65)33-38-24-26-39(86)27-25-38)53(90)76-35-49(87)77-47(57(94)82-43)34-37-15-5-4-6-16-37/h4-8,15-16,24-27,36,40-48,50,86H,3,9-14,17-23,28-35,64-65H2,1-2H3,(H2,66,88)(H,76,90)(H,77,87)(H,78,95)(H,79,89)(H,80,91)(H,81,92)(H,82,94)(H,83,96)(H,84,93)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t36-,40-,41-,42+,43-,44-,45-,46-,47-,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]Diprenorphine from rat kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Mus musculus (Mouse)) | BDBM50047457
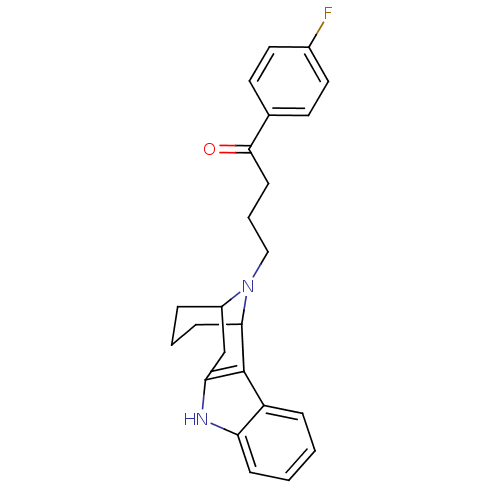
(4-[9,16-diazatetracyclo[10.3.1.02,10.03,8]hexadeca...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCCC1c1c(C2)[nH]c2ccccc12 |TLB:11:12:18.19.20:16.14.15,THB:21:19:12:16.14.15,27:18:12:16.14.15| Show InChI InChI=1S/C24H25FN2O/c25-17-12-10-16(11-13-17)23(28)9-4-14-27-18-5-3-8-22(27)24-19-6-1-2-7-20(19)26-21(24)15-18/h1-2,6-7,10-13,18,22,26H,3-5,8-9,14-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50244168

(CHEMBL4073496 | US10533010, Example I-239 | US1120...)Show SMILES Cc1nn(C)c(C)c1-c1c(Cl)ccc2c(CCCOc3cc(C)c(Cl)c(C)c3)c3C(=O)N(CCCn3c12)c1cc(C(O)=O)c2ccn(C)c2c1 |(8.69,-5.59,;7.22,-5.12,;6.74,-3.66,;5.2,-3.66,;4.29,-2.42,;4.73,-5.13,;3.26,-5.61,;5.98,-6.03,;5.98,-7.57,;4.66,-8.35,;3.32,-7.58,;4.65,-9.89,;5.99,-10.66,;7.32,-9.89,;8.8,-10.37,;9.27,-11.83,;10.78,-12.15,;11.25,-13.62,;12.76,-13.94,;13.24,-15.4,;14.74,-15.71,;15.22,-17.17,;16.73,-17.49,;14.19,-18.33,;14.66,-19.79,;12.68,-18.01,;11.65,-19.15,;12.2,-16.54,;9.71,-9.11,;11.26,-9.17,;11.87,-10.59,;12.28,-8,;11.99,-6.47,;10.63,-5.73,;9.2,-6.35,;8.8,-7.86,;7.32,-8.34,;13.77,-8.4,;14.85,-7.31,;16.33,-7.7,;17.42,-6.61,;17.02,-5.13,;18.91,-7.01,;16.73,-9.2,;18.1,-9.9,;17.85,-11.43,;16.33,-11.66,;15.63,-13.03,;15.64,-10.28,;14.16,-9.88,)| Show InChI InChI=1S/C39H39Cl2N5O4/c1-21-17-26(18-22(2)35(21)41)50-16-7-9-28-29-10-11-31(40)34(33-23(3)42-44(6)24(33)4)36(29)46-14-8-13-45(38(47)37(28)46)25-19-30(39(48)49)27-12-15-43(5)32(27)20-25/h10-12,15,17-20H,7-9,13-14,16H2,1-6H3,(H,48,49) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-labeled BH3 peptide binding to human MCL1 expressed in Escherichia coli BL21 (DE3) incubated for 0.5 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00690
BindingDB Entry DOI: 10.7270/Q2HD80JM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human Bcl-xl expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant preincubated for 30 mins follow... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00690
BindingDB Entry DOI: 10.7270/Q2HD80JM |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human Bcl-2 expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant preincubated for 30 mins followe... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00690
BindingDB Entry DOI: 10.7270/Q2HD80JM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50078163
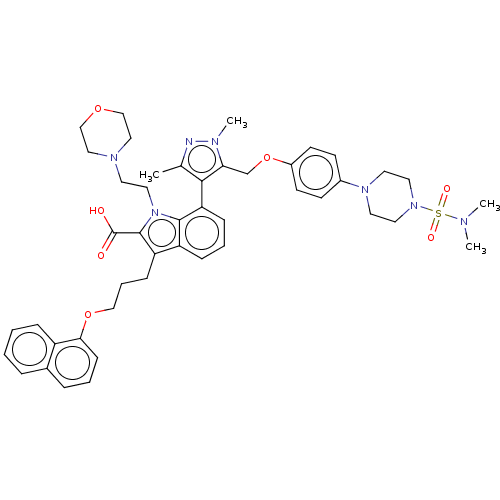
(CHEMBL3417704)Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)c1ccc(OCc2c(c(C)nn2C)-c2cccc3c(CCCOc4cccc5ccccc45)c(C(O)=O)n(CCN4CCOCC4)c23)cc1 |(-21.22,-16.71,;-19.99,-16.7,;-19.36,-17.76,;-19.23,-15.36,;-19.85,-14.3,;-20.46,-15.37,;-17.68,-15.35,;-16.91,-16.68,;-15.37,-16.67,;-14.6,-15.33,;-15.38,-14,;-16.92,-14.01,;-13.06,-15.32,;-12.28,-16.64,;-10.74,-16.63,;-9.98,-15.29,;-8.44,-15.28,;-7.69,-13.93,;-6.15,-13.92,;-5.26,-12.68,;-3.79,-13.11,;-2.81,-12.36,;-3.77,-14.65,;-5.22,-15.15,;-5.58,-16.33,;-5.74,-11.22,;-7.27,-10.9,;-7.75,-9.44,;-6.72,-8.27,;-5.21,-8.6,;-3.97,-7.71,;-3.98,-6.17,;-2.65,-5.4,;-2.66,-3.85,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,;-2.74,-8.57,;-1.29,-8.05,;-.35,-8.85,;-1.06,-6.84,;-3.2,-10.04,;-2.28,-11.27,;-.75,-11.09,;.17,-12.33,;1.7,-12.15,;2.62,-13.39,;2.01,-14.8,;.48,-14.98,;-.44,-13.74,;-4.73,-10.05,;-10.76,-13.96,;-12.3,-13.98,)| Show InChI InChI=1S/C46H55N7O7S/c1-33-43(41(49(4)47-33)32-60-36-19-17-35(18-20-36)51-22-24-52(25-23-51)61(56,57)48(2)3)40-14-8-13-38-39(15-9-29-59-42-16-7-11-34-10-5-6-12-37(34)42)45(46(54)55)53(44(38)40)26-21-50-27-30-58-31-28-50/h5-8,10-14,16-20H,9,15,21-32H2,1-4H3,(H,54,55) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human MCL1 expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant preincubated for 30 mins followed... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00690
BindingDB Entry DOI: 10.7270/Q2HD80JM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50581350
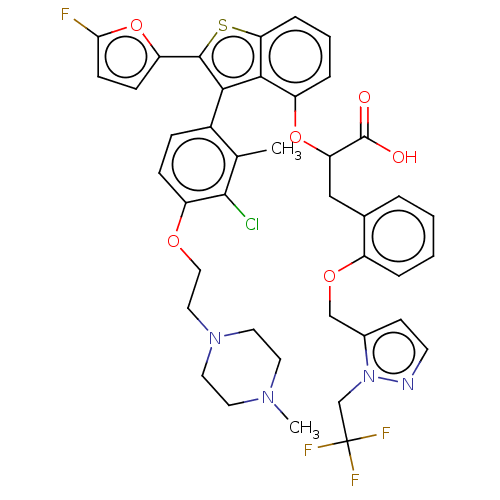
(CHEMBL5079948)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4cccc(OC(Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccc(F)o3)c(C)c2Cl)CC1 |(24.77,-9.63,;23.27,-9.95,;22.79,-11.42,;21.3,-11.74,;20.26,-10.6,;18.75,-10.93,;18.29,-12.39,;16.78,-12.73,;16.32,-14.19,;14.81,-14.53,;14.35,-15.99,;15.38,-17.13,;14.91,-18.59,;15.83,-19.83,;14.93,-21.08,;13.46,-20.61,;12.12,-21.39,;10.78,-20.62,;10.78,-19.08,;12.11,-18.31,;12.11,-16.77,;10.77,-16,;10.77,-14.46,;9.43,-13.7,;8.11,-14.48,;6.77,-13.72,;6.76,-12.17,;8.1,-11.4,;9.43,-12.16,;10.76,-11.39,;10.76,-9.85,;9.43,-9.08,;8.02,-9.71,;6.99,-8.57,;7.76,-7.23,;9.27,-7.55,;10.41,-6.52,;11.87,-6.99,;12.96,-8.08,;11.47,-8.48,;13.02,-5.96,;9.44,-16.78,;8.1,-16.01,;9.44,-18.32,;13.45,-19.07,;17.37,-19.82,;18.27,-18.57,;19.73,-19.03,;19.74,-20.57,;21,-21.47,;18.28,-21.06,;16.89,-16.8,;17.92,-17.94,;17.36,-15.33,;18.86,-15.01,;20.72,-9.14,;22.23,-8.81,)| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human MCL1 (173 to 321 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant incubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00690
BindingDB Entry DOI: 10.7270/Q2HD80JM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50047436
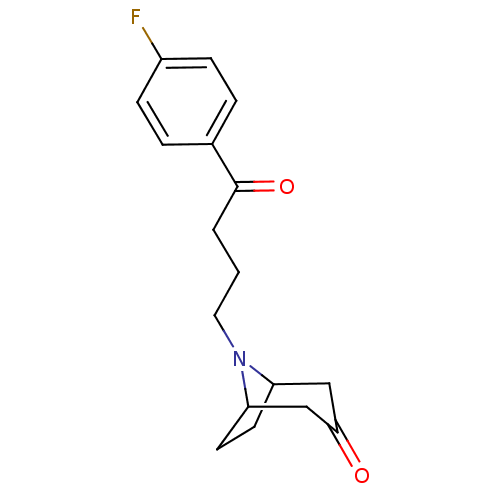
(8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-8-aza-bicyclo[...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1CC(=O)C2 |THB:19:18:12:14.15| Show InChI InChI=1S/C17H20FNO2/c18-13-5-3-12(4-6-13)17(21)2-1-9-19-14-7-8-15(19)11-16(20)10-14/h3-6,14-15H,1-2,7-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846
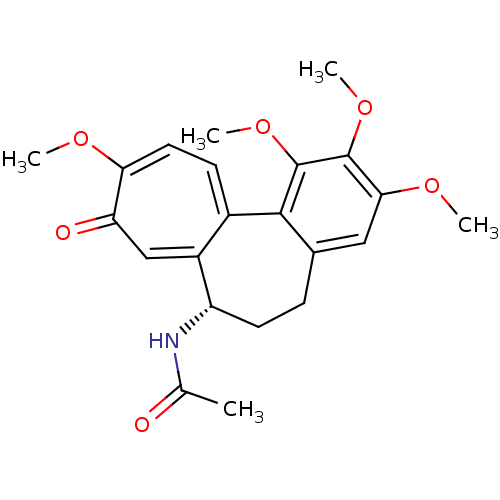
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM239078

(US9416103, TV-5-157)Show InChI InChI=1S/C24H23NO2/c1-3-4-15-25-16-21(19-12-8-14-22(27-2)23(19)25)24(26)20-13-7-10-17-9-5-6-11-18(17)20/h5-14,16H,3-4,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas
US Patent
| Assay Description
A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... |
US Patent US9416103 (2016)
BindingDB Entry DOI: 10.7270/Q2HX1BKQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50036647
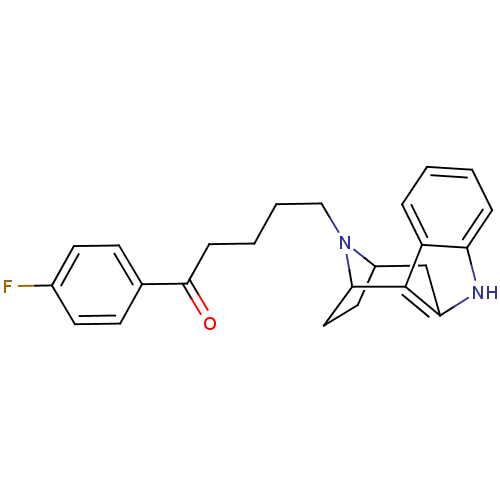
((7R,10S)-5-[9,15-diazatetracyclo[10.2.1.02,10.03,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCCN1C2CCC1c1c(C2)[nH]c2ccccc12 |TLB:12:13:18.19.20:16.15,THB:21:19:13:16.15,27:18:13:16.15| Show InChI InChI=1S/C24H25FN2O/c25-17-10-8-16(9-11-17)23(28)7-3-4-14-27-18-12-13-22(27)24-19-5-1-2-6-20(19)26-21(24)15-18/h1-2,5-6,8-11,18,22,26H,3-4,7,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine receptor D2 in rat striatal membranes by [3H]-sulpiride displacement. |
J Med Chem 36: 3073-6 (1993)
BindingDB Entry DOI: 10.7270/Q2RF5T2N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Mus musculus (Mouse)) | BDBM239078

(US9416103, TV-5-157)Show InChI InChI=1S/C24H23NO2/c1-3-4-15-25-16-21(19-12-8-14-22(27-2)23(19)25)24(26)20-13-7-10-17-9-5-6-11-18(17)20/h5-14,16H,3-4,15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... |
J Med Chem 56: 4537-50 (2013)
Article DOI: 10.1021/jm400268b
BindingDB Entry DOI: 10.7270/Q25M68M1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A/2B/2C
(Mus musculus (Mouse)) | BDBM50036648

((7R,10S)-4-[5-fluoro-9,15-diazatetracyclo[10.2.1.0...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1C2CCC1c1c(C2)[nH]c2ccc(F)cc12 |TLB:11:12:17.18.19:15.14,THB:27:17:12:15.14,20:18:12:15.14| Show InChI InChI=1S/C23H22F2N2O/c24-15-5-3-14(4-6-15)22(28)2-1-11-27-17-8-10-21(27)23-18-12-16(25)7-9-19(18)26-20(23)13-17/h3-7,9,12,17,21,26H,1-2,8,10-11,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 2 receptor from rat cortical synaptosomal membrane using radioligand [3H]ketanserin. |
J Med Chem 36: 1488-95 (1993)
BindingDB Entry DOI: 10.7270/Q2KD1X0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5619-25 (2009)
Article DOI: 10.1021/jm900577k
BindingDB Entry DOI: 10.7270/Q2QN66T6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data