

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110268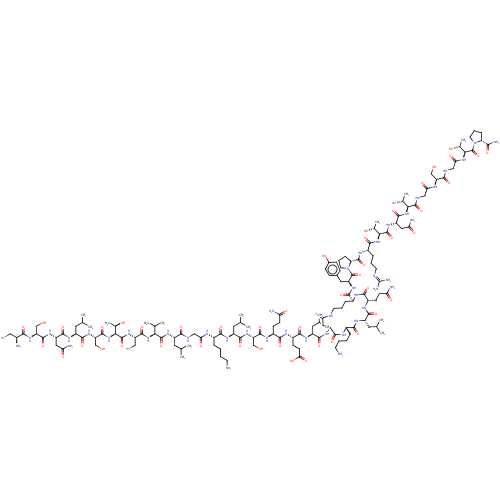 (CHEMBL2369895 | CSNLSTCVLGKLSQELc[DKLQK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50024170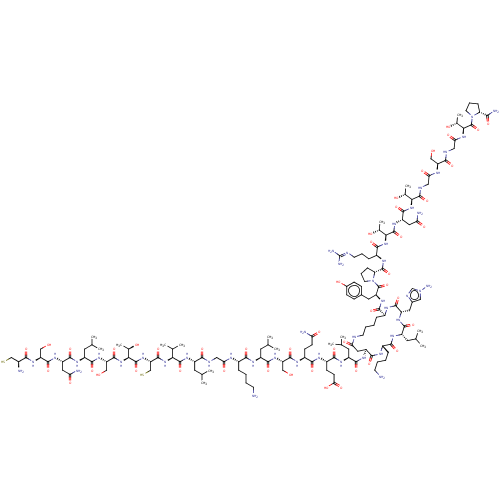 (CHEMBL2369912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110272 (CHEMBL2369907 | CSNLSTCVLGKLSQELc[DKLHK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110275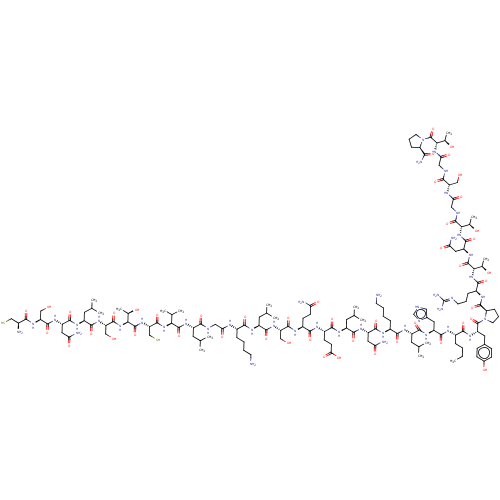 (CGNLSTCBLGTYTQDF[DKFHO]YPQTAIGVGAP-amide | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110265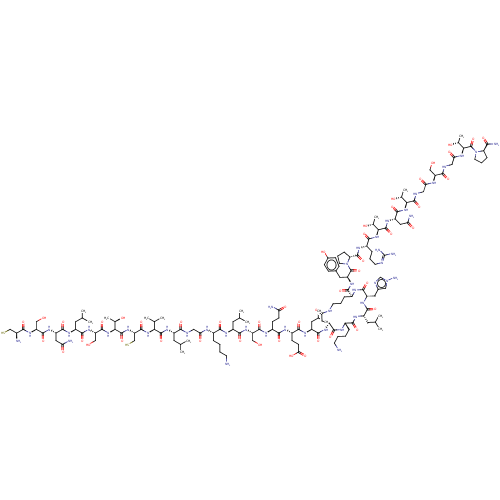 (CHEMBL2369886 | CSNLSTCVLGKLSQELc[DKLHO]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110273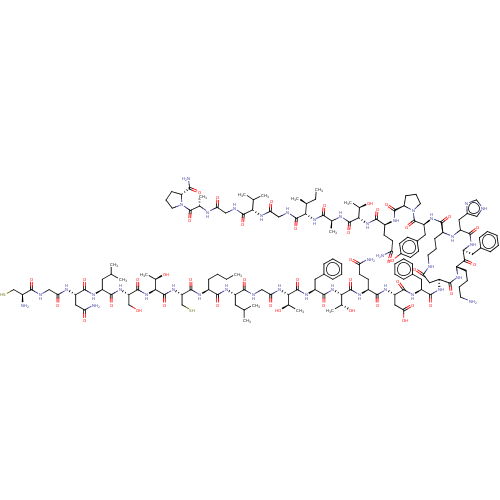 (CGNLSTCMLGTYTQDFc[DKFHK]FPQTAIGVGAP-amide | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29864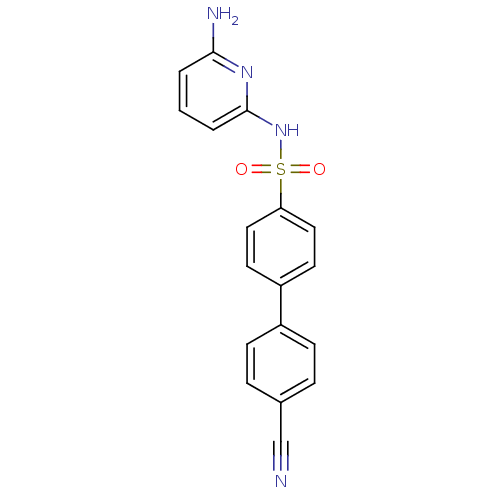 (N-(Pyridin-2-yl) arylsulfonamide, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29863 (N-(Pyridin-2-yl) arylsulfonamide, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29862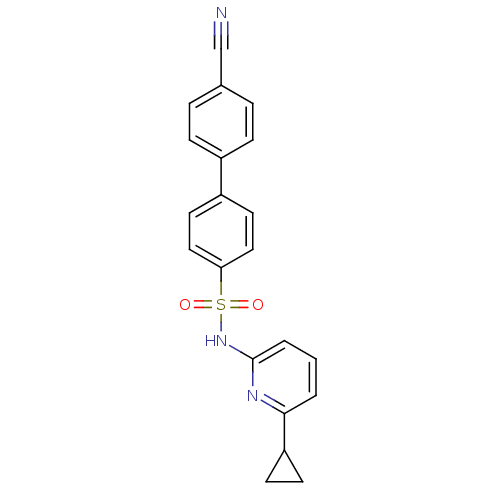 (N-(Pyridin-2-yl) arylsulfonamide, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29860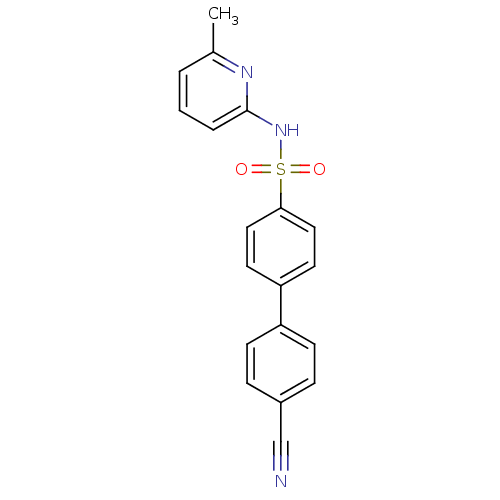 (N-(Pyridin-2-yl) arylsulfonamide, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29866 (N-(Pyridin-2-yl) arylsulfonamide, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110267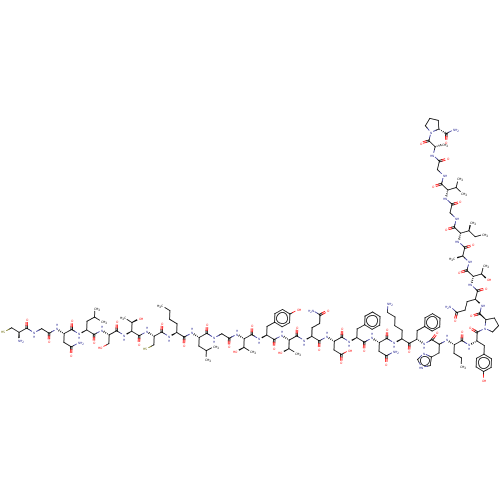 (CGNLSTCBLGTYTQDFNKFHZYPQTAIGVGAP-amide | CHEMBL236...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231651 (CHEMBL283398) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29858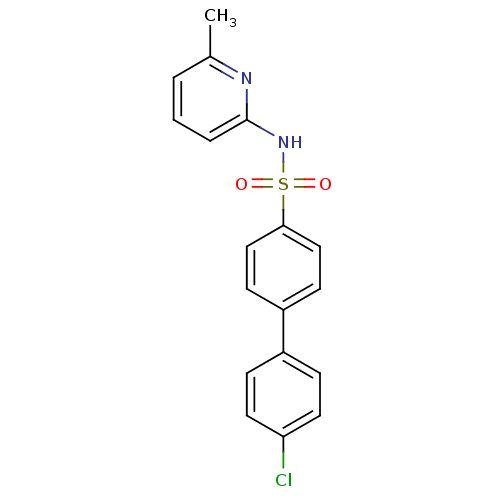 (N-(Pyridin-2-yl) arylsulfonamide, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29861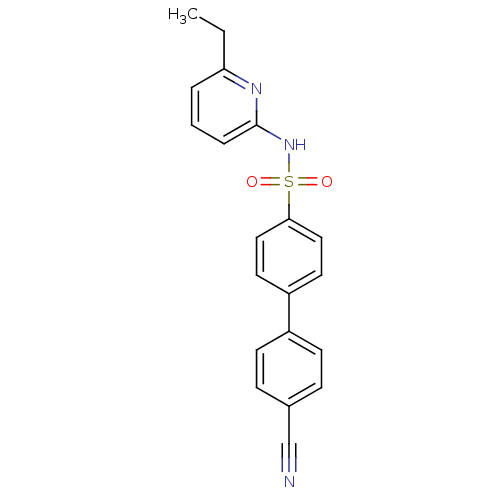 (N-(Pyridin-2-yl) arylsulfonamide, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29865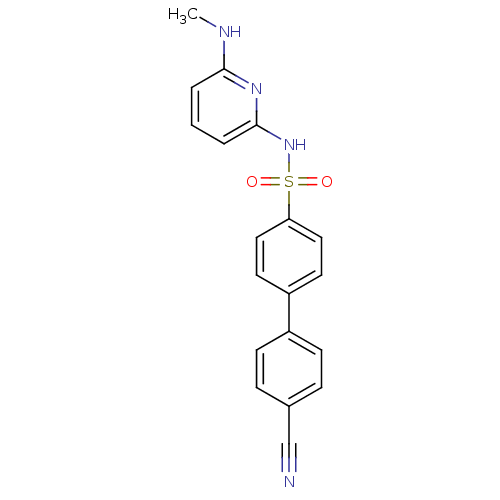 (N-(Pyridin-2-yl) arylsulfonamide, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.80 | -45.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50209178 (5-Bromo-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM81498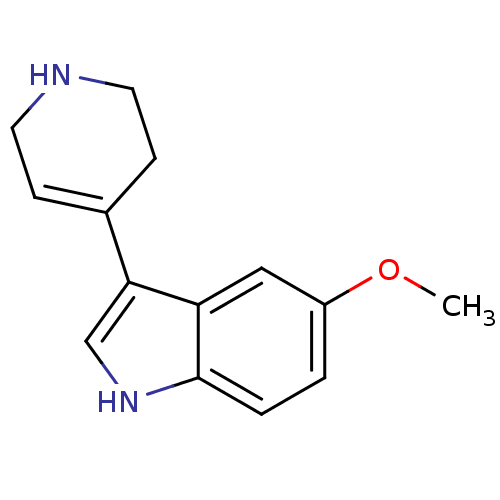 (5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50158037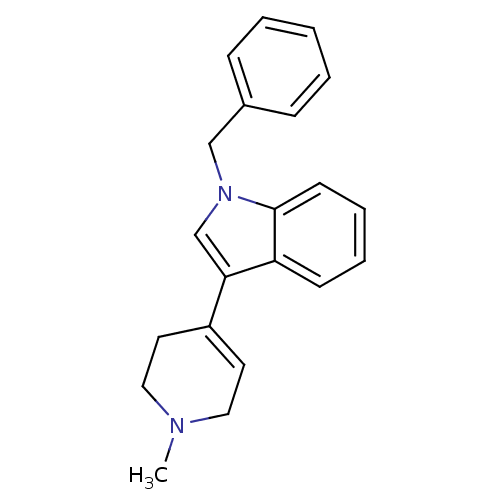 (1-Benzyl-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231649 (CHEMBL281432) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50231652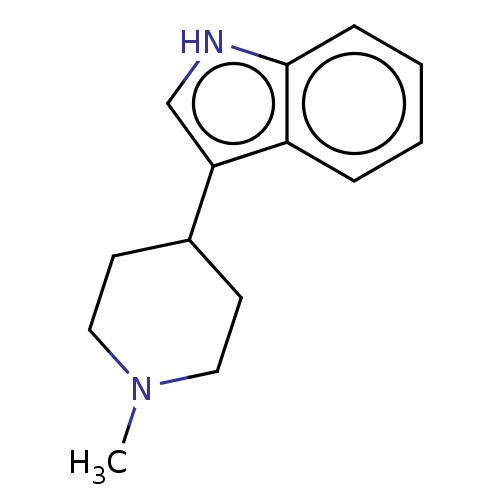 (CHEMBL341485) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231634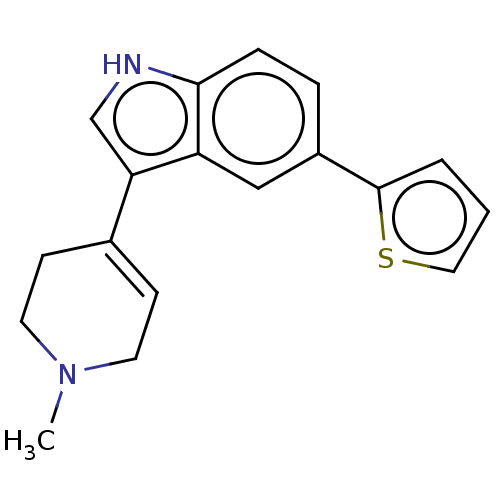 (CHEMBL27643) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231669 (CHEMBL27719) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29857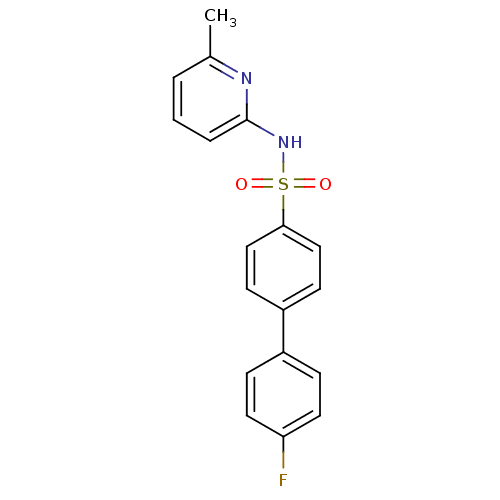 (N-(Pyridin-2-yl) arylsulfonamide, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50209169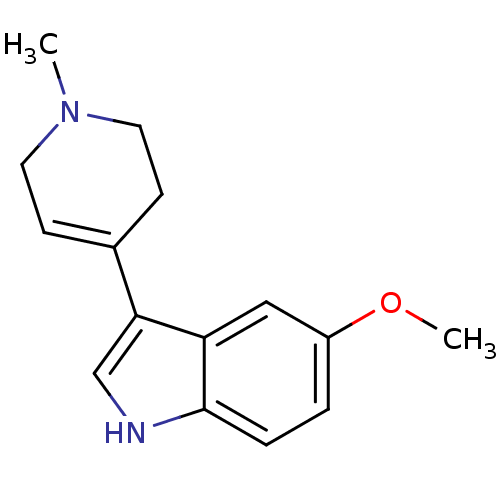 (5-Methoxy-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50209179 (3-(1-Methyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-nit...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231662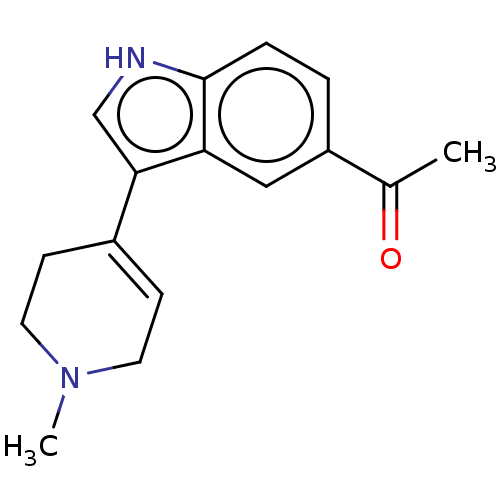 (CHEMBL281434) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231661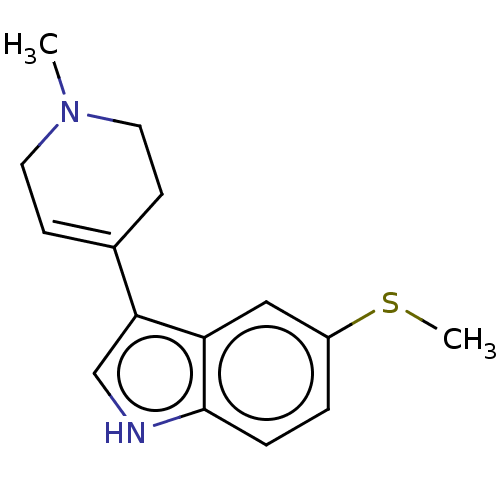 (CHEMBL287576) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231639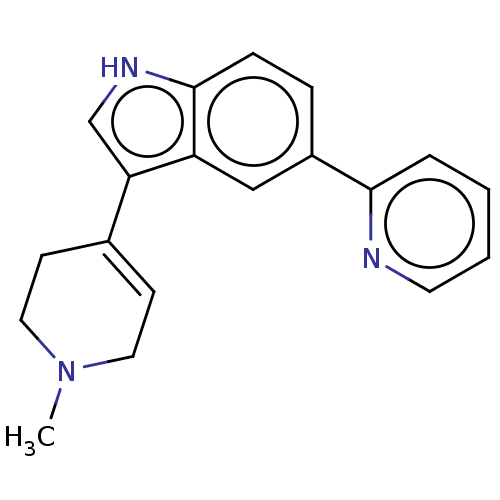 (CHEMBL26900) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231663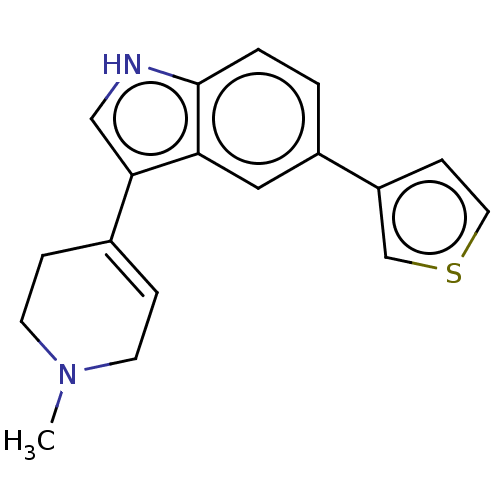 (CHEMBL417470) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231643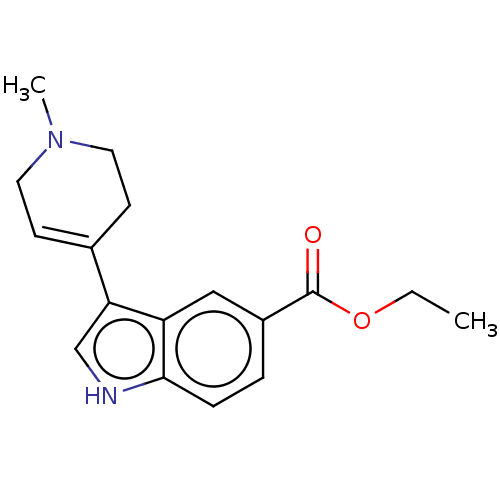 (CHEMBL26311) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231636 (CHEMBL287097) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110271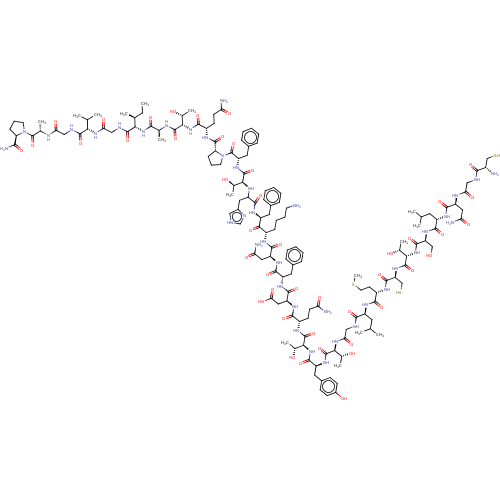 (CGNLSTCMLGTYTQDFNKFHTFPQTAIGVGAP-amide | CHEMBL236...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50231640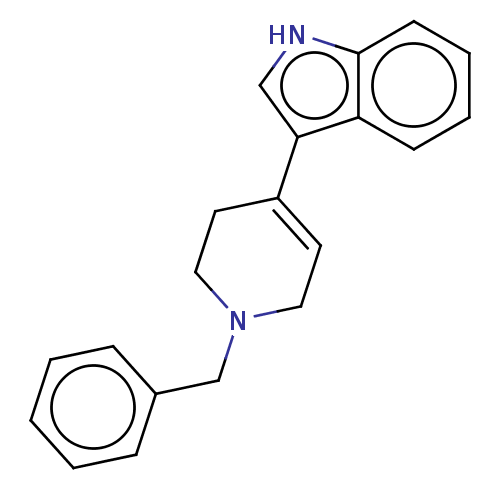 (CHEMBL285157 | TCMDC-123457) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50209163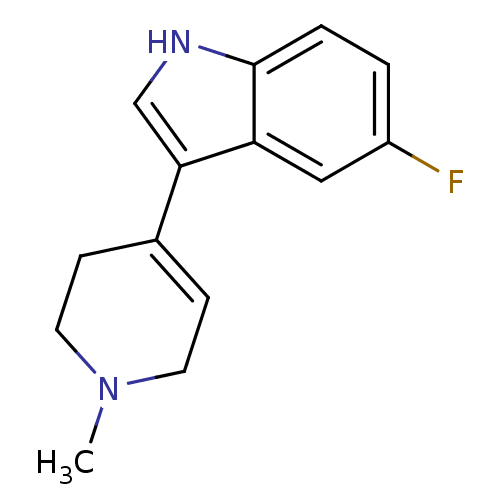 (5-Fluoro-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50209178 (5-Bromo-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29856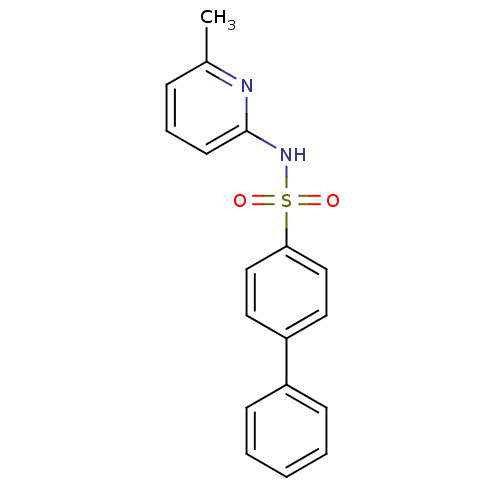 (N-(Pyridin-2-yl) arylsulfonamide, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | -41.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50209174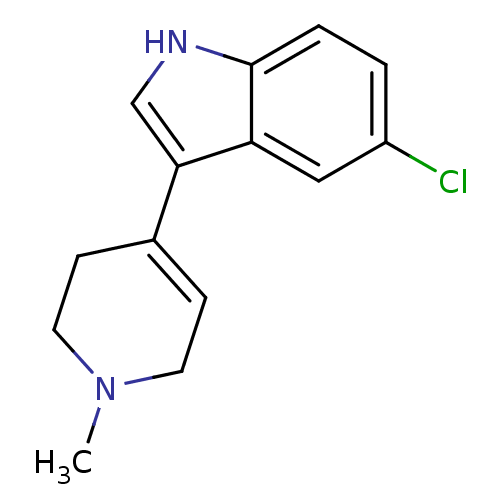 (5-Chloro-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231632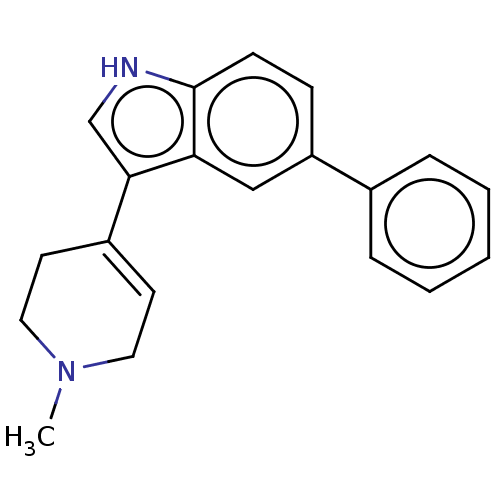 (CHEMBL284107 | TCMDC-138962) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50209177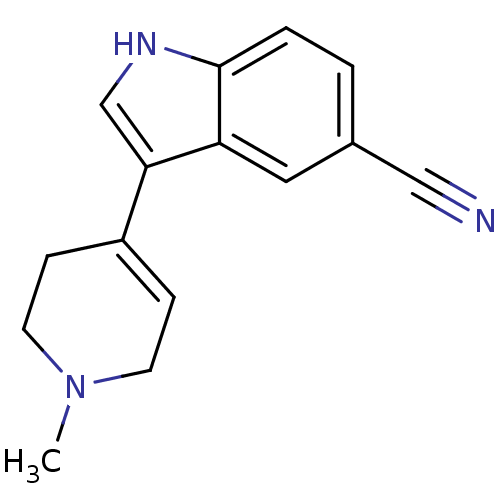 (3-(1-Methyl-1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231665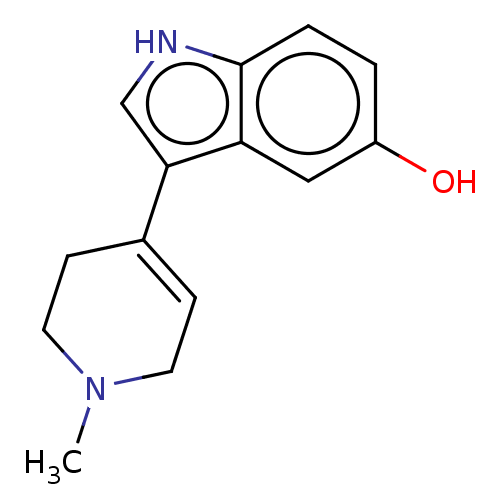 (CHEMBL26836) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29859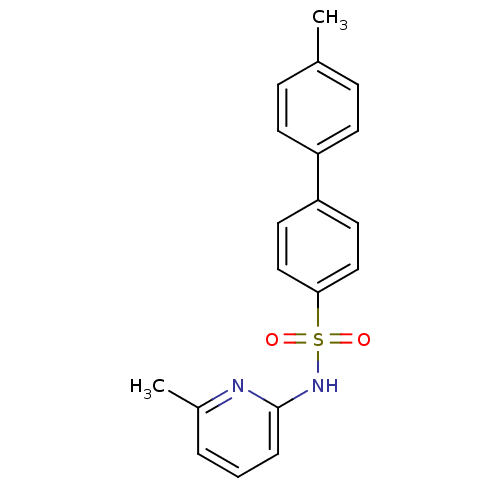 (N-(Pyridin-2-yl) arylsulfonamide, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50015718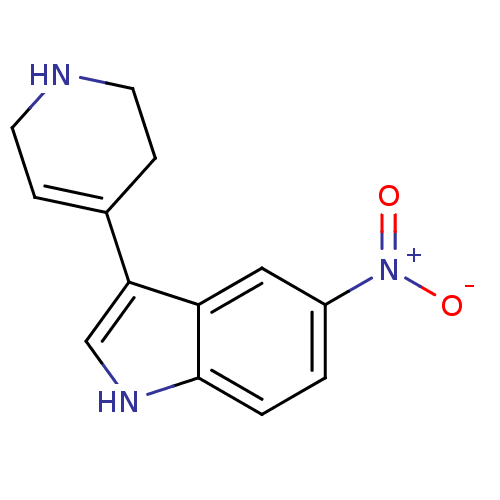 (5-Nitro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50209163 (5-Fluoro-3-(1-methyl-1,2,3,6-tetrahydro-pyridin-4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM31034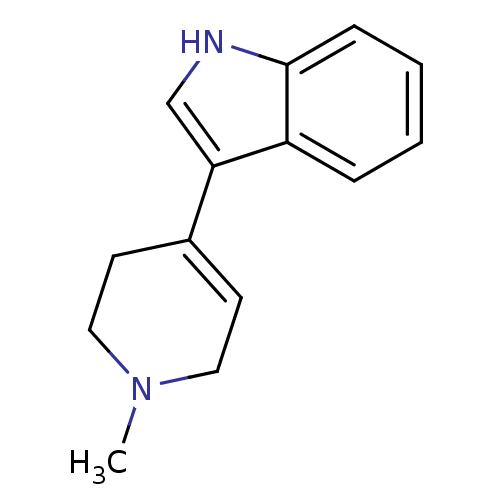 (3-(1-methyl-3,6-dihydro-2H-pyridin-4-yl)-1H-indole...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor using [3H]-ketanserin as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231653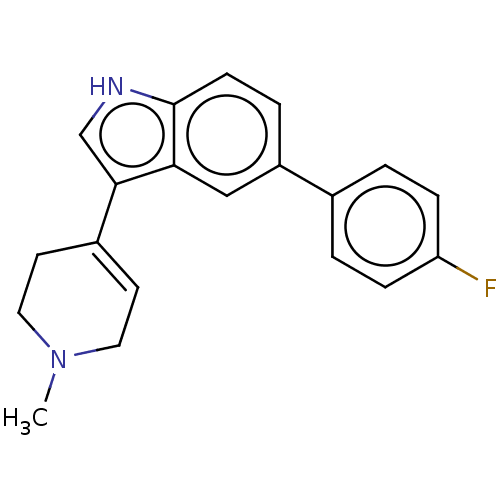 (CHEMBL26972) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231659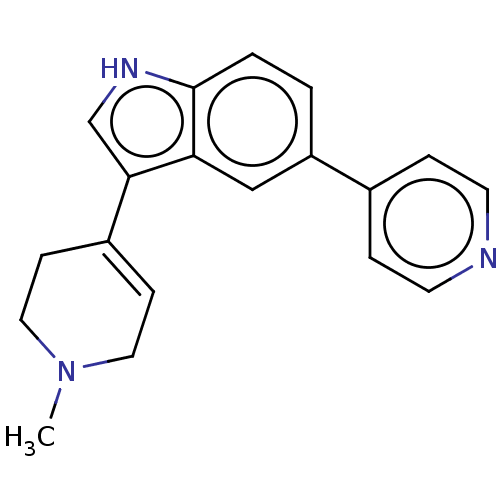 (CHEMBL282799) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231660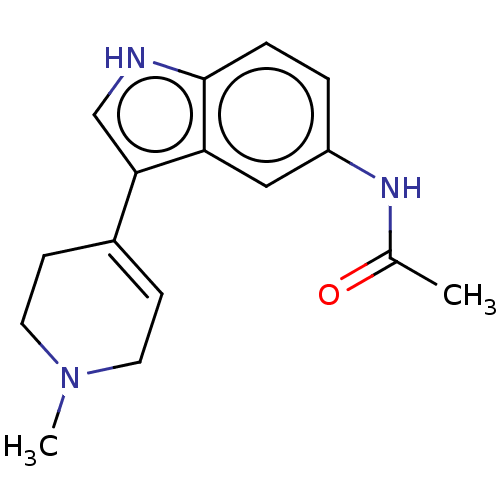 (CHEMBL26322) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50231700 (CHEMBL27998) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]8-OH-DPAT as radioligand. | J Med Chem 36: 4006-14 (1993) Article DOI: 10.1021/jm00077a003 BindingDB Entry DOI: 10.7270/Q2W37ZJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM29854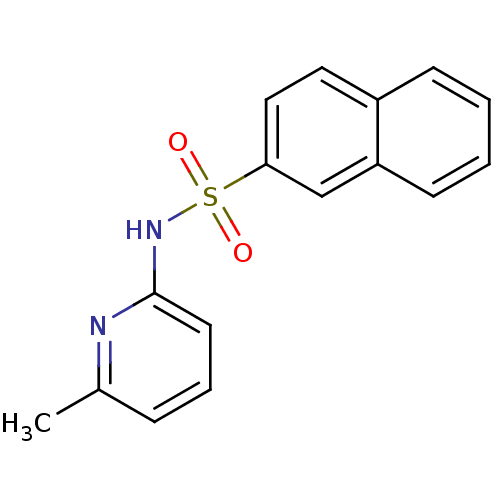 (N-(Pyridin-2-yl) arylsulfonamide, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 84 | -40.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer | Assay Description The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... | Bioorg Med Chem Lett 19: 3493-7 (2009) Article DOI: 10.1016/j.bmcl.2009.05.011 BindingDB Entry DOI: 10.7270/Q2SB4428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 583 total ) | Next | Last >> |