

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50407800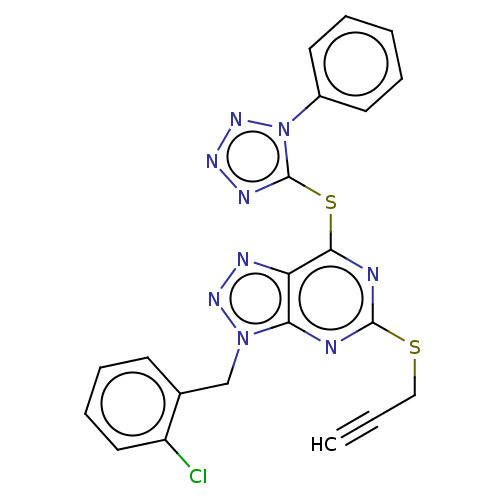 (CHEMBL5291138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346870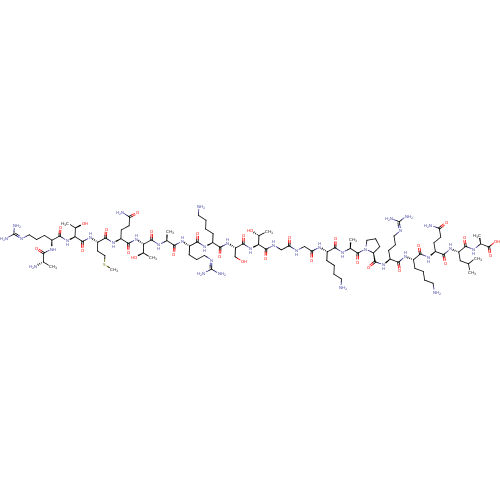 (CHEMBL1797647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346870 (CHEMBL1797647) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50586363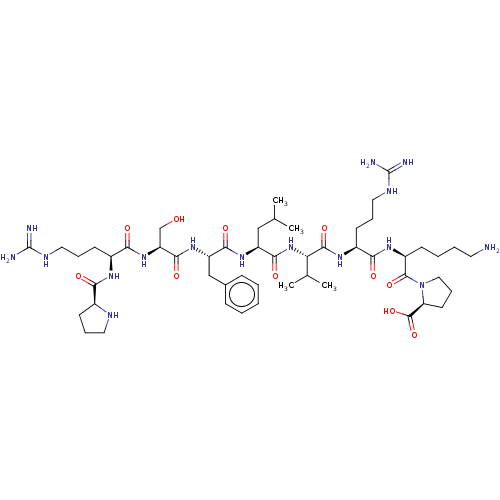 (CHEMBL5079374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50407795 (CHEMBL5281080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50407796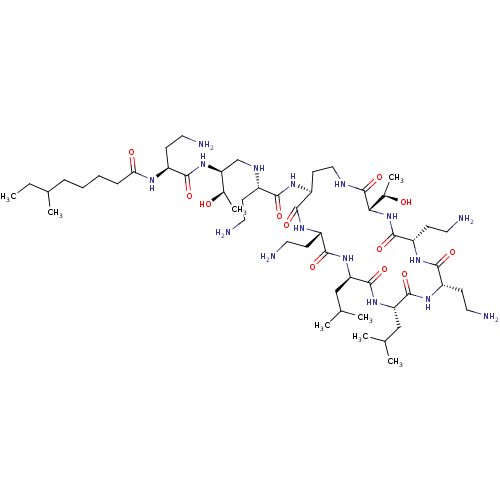 (CHEMBL1255711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50407722 (CHEMBL5287743) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568522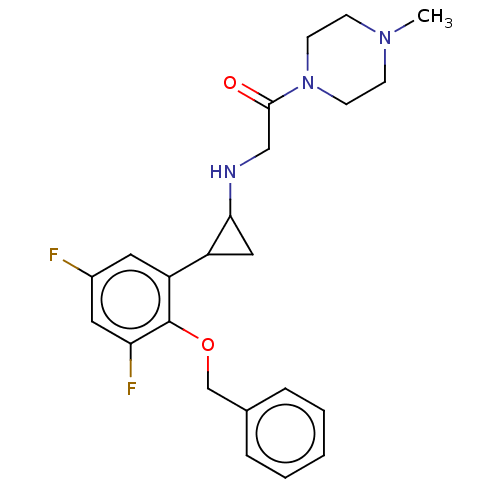 (CHEMBL4864352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346586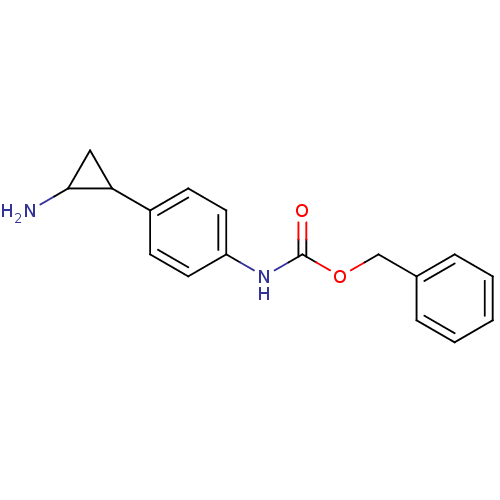 (CHEMBL1795981 | US8765820, 5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568521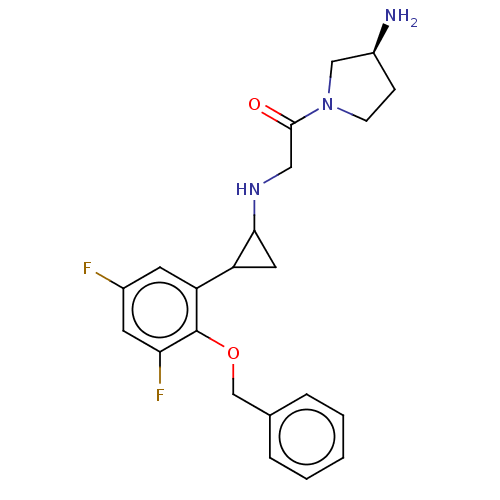 (CHEMBL4878787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346585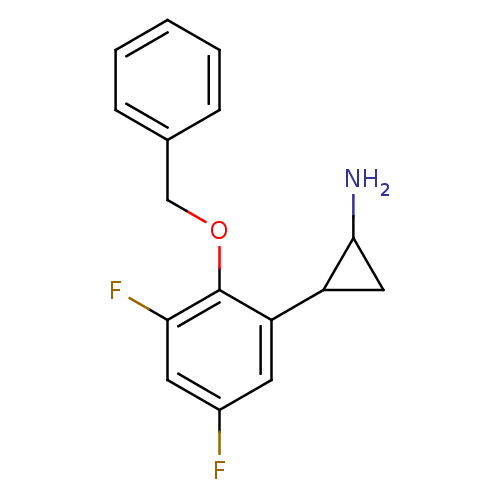 (CHEMBL1795980 | S2101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 833 residues) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346863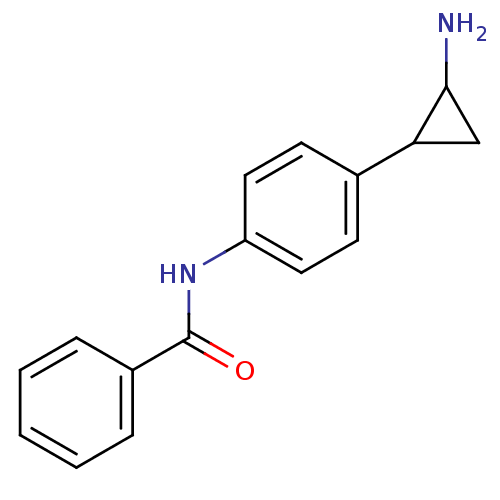 (CHEMBL1797640 | US8765820, 5b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346864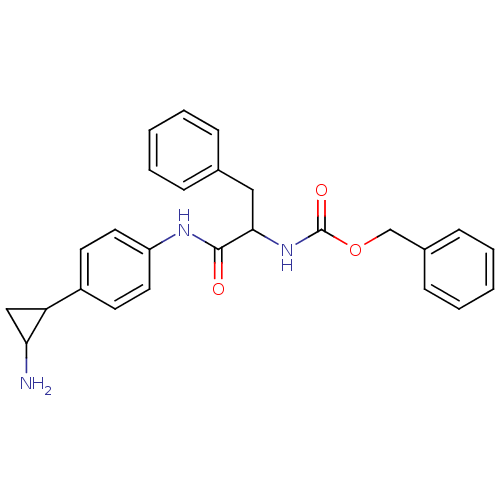 (CHEMBL1797641 | CHEMBL3104337 | US8765820, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346864 (CHEMBL1797641 | CHEMBL3104337 | US8765820, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50158869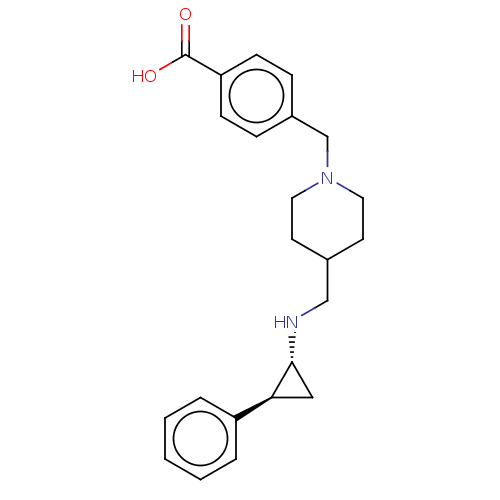 (CHEMBL3786182 | US10836743, Compound GSK-2879552 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LSD1 (unknown origin) using H3K4(diMe) peptide as substrate measured after 60 mins by amplex red dye based HRP-coupled assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50407723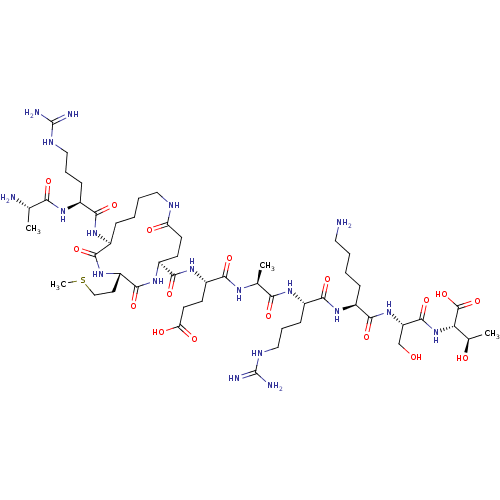 (CHEMBL5274037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Mean functional activity against human H3 receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346863 (CHEMBL1797640 | US8765820, 5b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-A expressed in Pichia pastoris using kynuramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346866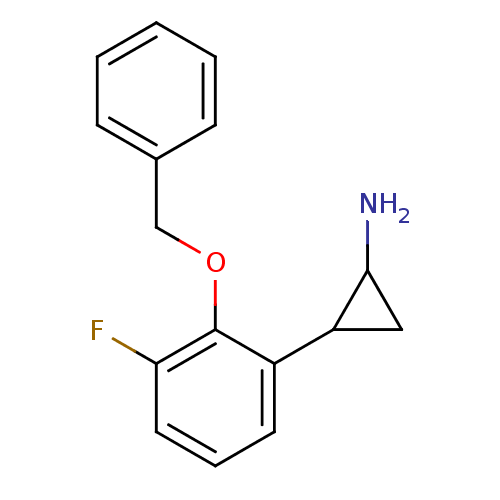 (CHEMBL1797643 | S1201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346863 (CHEMBL1797640 | US8765820, 5b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-B expressed in Pichia pastoris using benzylamine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MAO-B expressed in Pichia pastoris using benzylamine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346865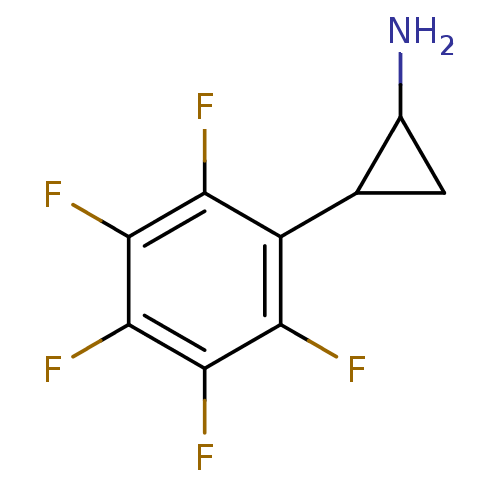 (2-PFPA | CHEMBL1797642) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in baculovirus infected BTI insect cells using tyramine as substrate by 4-AAP/3,5-DCHBS and amplex red dye based ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346865 (2-PFPA | CHEMBL1797642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hexahistidine-tagged human LSD1 (172 to 833 residues) expressed in Escherichia coli Rosetta (DE3) cells using H3K4me2 peptide as substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1B (Mus musculus) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50407721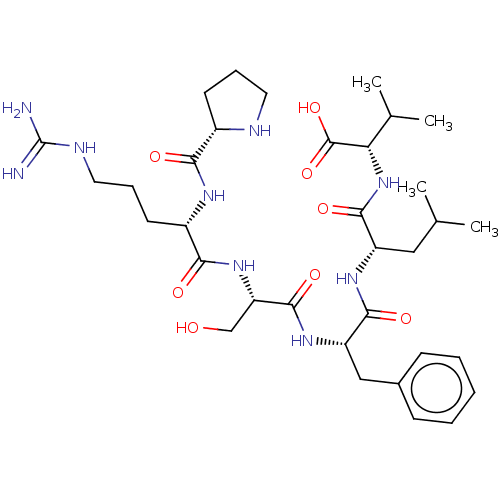 (CHEMBL5266466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by fluore... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1B (Mus musculus) | BDBM50346864 (CHEMBL1797641 | CHEMBL3104337 | US8765820, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50346866 (CHEMBL1797643 | S1201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1B (Mus musculus) | BDBM50346863 (CHEMBL1797640 | US8765820, 5b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346866 (CHEMBL1797643 | S1201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by peroxidase-coupled ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells using tyramine as substrate by 4-AAP/3,5-DCHBS and amplex red dye based ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal 6histidine-tagged LSD1 (unknown origin) expressed in Escherichia coli BL21(DE3) cells assessed as reduction in Peroxide prod... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 2 (Homo sapiens (Human)) | BDBM50568521 (CHEMBL4878787) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LSD2 (unknown origin) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 2 (Homo sapiens (Human)) | BDBM50346585 (CHEMBL1795980 | S2101) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LSD2 (unknown origin) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 2 (Homo sapiens (Human)) | BDBM50568522 (CHEMBL4864352) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LSD2 (unknown origin) using Lys4-dimethylated H3 (1-20) peptide as substrate incubated for 30 mins by peroxidase-coupled assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50568521 (CHEMBL4878787) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by fluore... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50568521 (CHEMBL4878787) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by peroxidase-coupled ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50568522 (CHEMBL4864352) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by fluore... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50568522 (CHEMBL4864352) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells using tyramine as substrate incubated for 30 mins by peroxidase-coupled ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50346865 (2-PFPA | CHEMBL1797642) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A expressed in expressed in baculovirus infected BTI insect cells using tyramine as substrate by peroxidase-coupled method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568531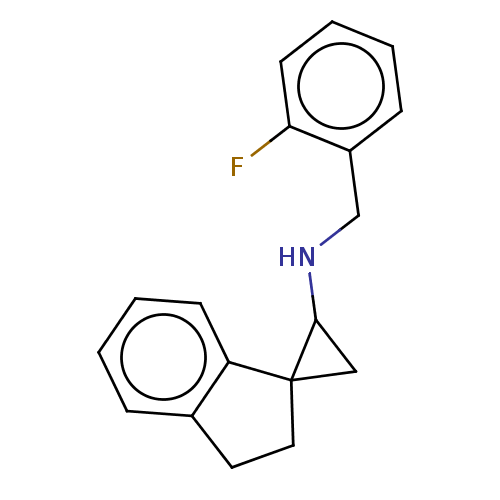 (CHEMBL4855475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 852 residues) using Bio-H3K4me2 (1 to 24 residues) as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 1 (Homo sapiens (Human)) | BDBM50568523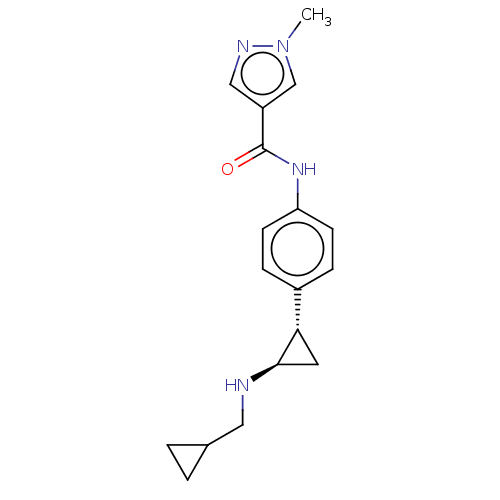 (CHEMBL4875015) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal His-SUMO tagged human LSD1 (172 to 852 residues)/His-tagged CoREST (286 to 482 residues) expressed in Escherichia coli BL21 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568527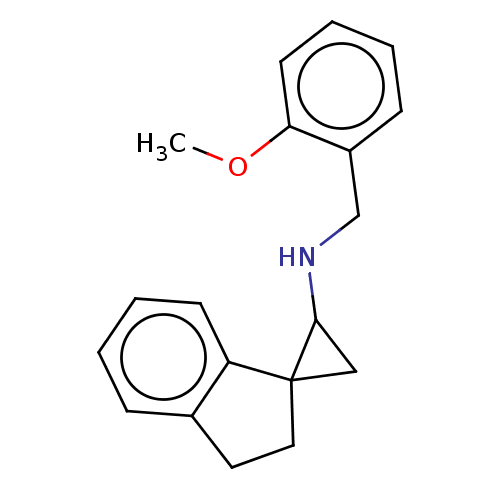 (CHEMBL4861378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 852 residues) using Bio-H3K4me2 (1 to 24 residues) as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568529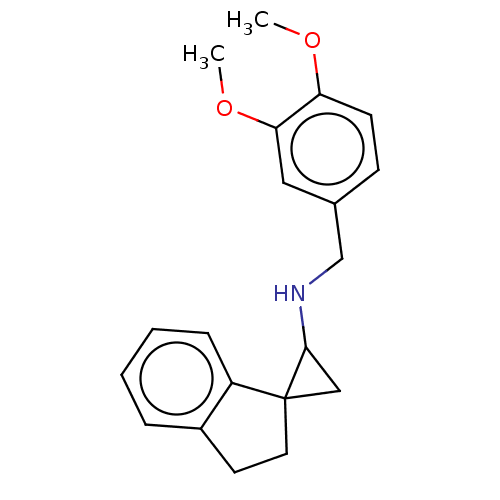 (CHEMBL4851339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 (172 to 852 residues) using Bio-H3K4me2 (1 to 24 residues) as substrate incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50568520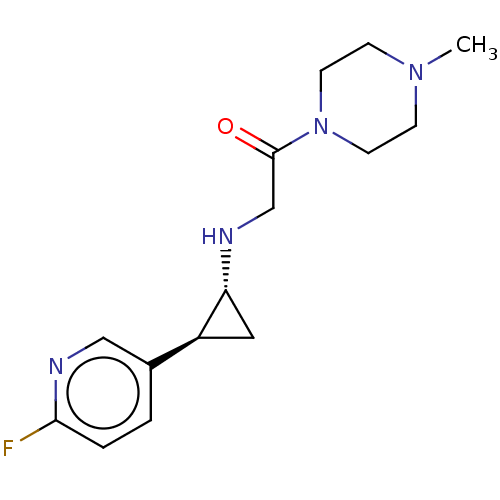 (CHEMBL4851520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human LSD1 (158 to end residues) expressed in Escherichia coli using biotinylated H3K4Me2 peptide as substrate pr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00919 BindingDB Entry DOI: 10.7270/Q28K7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |