 Found 2123 hits with Last Name = 'iura' and Initial = 'y'
Found 2123 hits with Last Name = 'iura' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513317
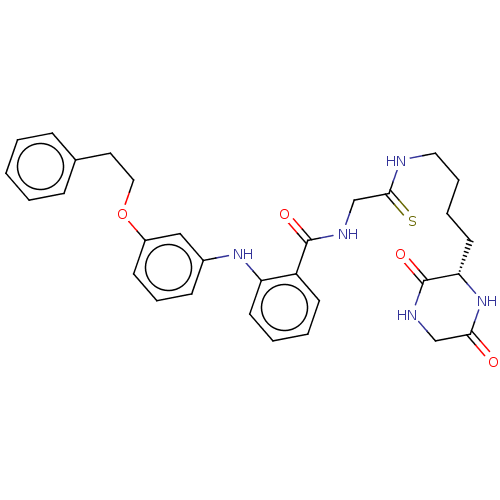
(CHEMBL4465620)Show SMILES O=C(NCC(=S)NCCCC[C@@H]1NC(=O)CNC1=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1 |r| Show InChI InChI=1S/C31H35N5O4S/c37-28-20-33-31(39)27(36-28)15-6-7-17-32-29(41)21-34-30(38)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)40-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,41)(H,33,39)(H,34,38)(H,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513318
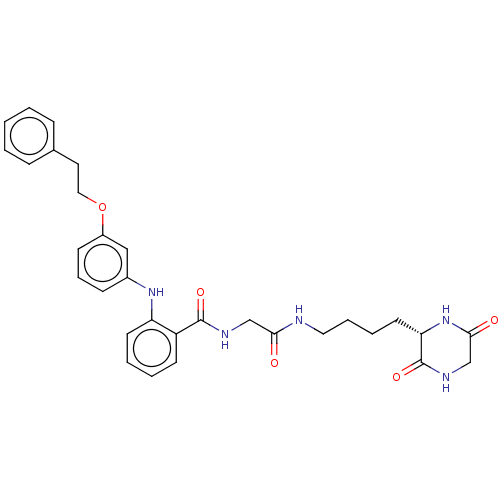
(CHEMBL4516553)Show SMILES O=C(CNC(=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1)NCCCC[C@@H]1NC(=O)CNC1=O |r| Show InChI InChI=1S/C31H35N5O5/c37-28(32-17-7-6-15-27-31(40)34-21-29(38)36-27)20-33-30(39)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)41-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,37)(H,33,39)(H,34,40)(H,36,38)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50524981
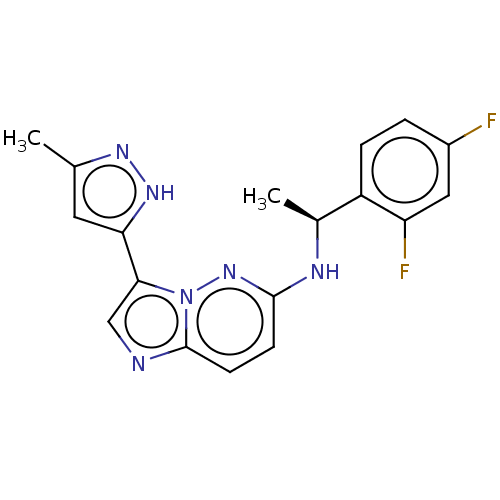
(CHEMBL4562879)Show SMILES C[C@H](Nc1ccc2ncc(-c3cc(C)n[nH]3)n2n1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C18H16F2N6/c1-10-7-15(24-23-10)16-9-21-18-6-5-17(25-26(16)18)22-11(2)13-4-3-12(19)8-14(13)20/h3-9,11H,1-2H3,(H,22,25)(H,23,24)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... |
J Med Chem 62: 4915-4935 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01630
BindingDB Entry DOI: 10.7270/Q2B56P57 |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461646
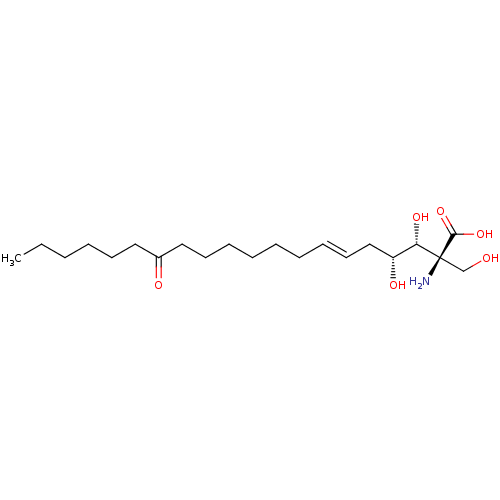
(CHEBI:582124 | Myriocin)Show SMILES CCCCCCC(=O)CCCCCC\C=C\C[C@@H](O)[C@H](O)[C@@](N)(CO)C(O)=O |r| Show InChI InChI=1S/C21H39NO6/c1-2-3-4-10-13-17(24)14-11-8-6-5-7-9-12-15-18(25)19(26)21(22,16-23)20(27)28/h9,12,18-19,23,25-26H,2-8,10-11,13-16,22H2,1H3,(H,27,28)/b12-9+/t18-,19+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390219
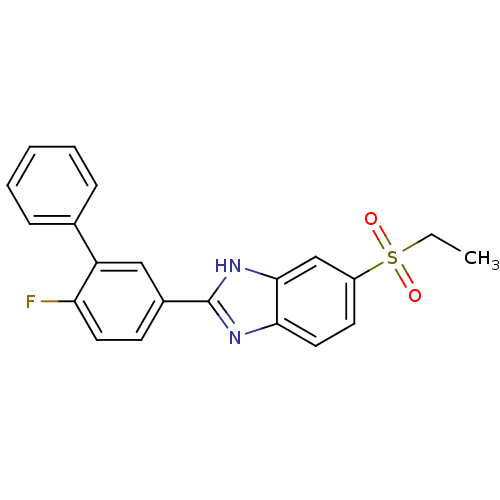
(CHEMBL2070148)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(F)c(c1)-c1ccccc1 Show InChI InChI=1S/C21H17FN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)15-8-10-18(22)17(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390216

(CHEMBL2070151)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(Cl)c(c1)-c1ccccc1 Show InChI InChI=1S/C21H17ClN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)15-8-10-18(22)17(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50285779
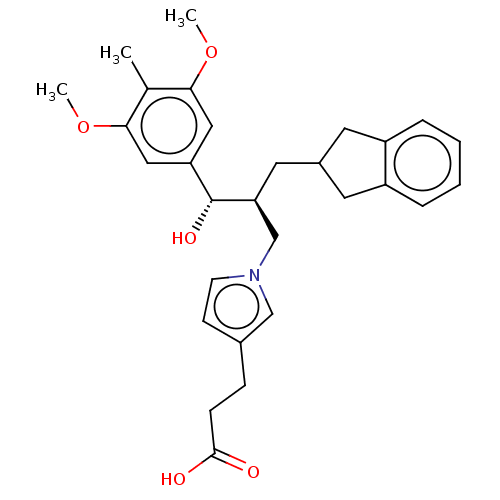
(CHEMBL4165205)Show SMILES COc1cc(cc(OC)c1C)[C@@H](O)[C@@H](CC1Cc2ccccc2C1)Cn1ccc(CCC(O)=O)c1 |r| Show InChI InChI=1S/C29H35NO5/c1-19-26(34-2)15-24(16-27(19)35-3)29(33)25(14-21-12-22-6-4-5-7-23(22)13-21)18-30-11-10-20(17-30)8-9-28(31)32/h4-7,10-11,15-17,21,25,29,33H,8-9,12-14,18H2,1-3H3,(H,31,32)/t25-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... |
ACS Med Chem Lett 8: 1281-1286 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00383
BindingDB Entry DOI: 10.7270/Q2PG1V8Z |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50285779

(CHEMBL4165205)Show SMILES COc1cc(cc(OC)c1C)[C@@H](O)[C@@H](CC1Cc2ccccc2C1)Cn1ccc(CCC(O)=O)c1 |r| Show InChI InChI=1S/C29H35NO5/c1-19-26(34-2)15-24(16-27(19)35-3)29(33)25(14-21-12-22-6-4-5-7-23(22)13-21)18-30-11-10-20(17-30)8-9-28(31)32/h4-7,10-11,15-17,21,25,29,33H,8-9,12-14,18H2,1-3H3,(H,31,32)/t25-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... |
ACS Med Chem Lett 8: 1281-1286 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00383
BindingDB Entry DOI: 10.7270/Q2PG1V8Z |
More data for this
Ligand-Target Pair | |
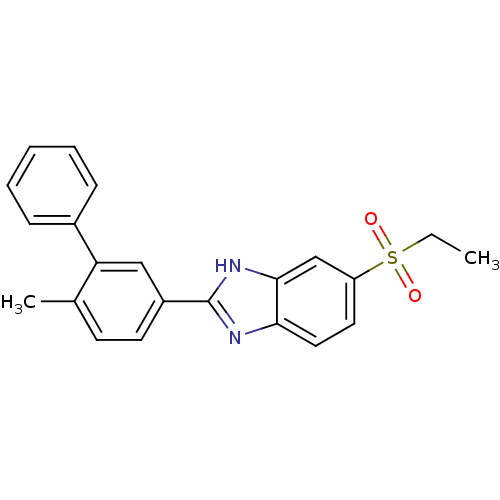
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390213
(CHEMBL2069316)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(C)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O2S/c1-3-27(25,26)18-11-12-20-21(14-18)24-22(23-20)17-10-9-15(2)19(13-17)16-7-5-4-6-8-16/h4-14H,3H2,1-2H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
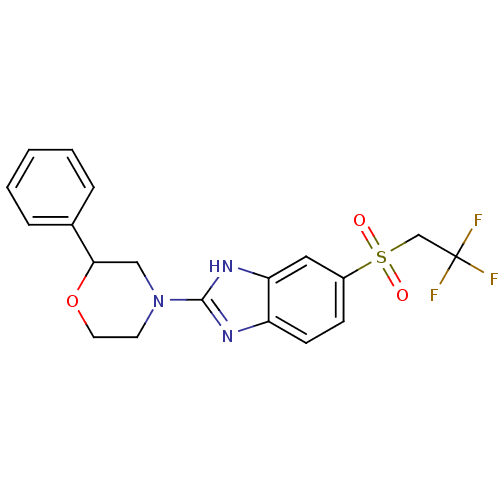
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394224
(CHEMBL2159167)Show SMILES FC(F)(F)CS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H18F3N3O3S/c20-19(21,22)12-29(26,27)14-6-7-15-16(10-14)24-18(23-15)25-8-9-28-17(11-25)13-4-2-1-3-5-13/h1-7,10,17H,8-9,11-12H2,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529296
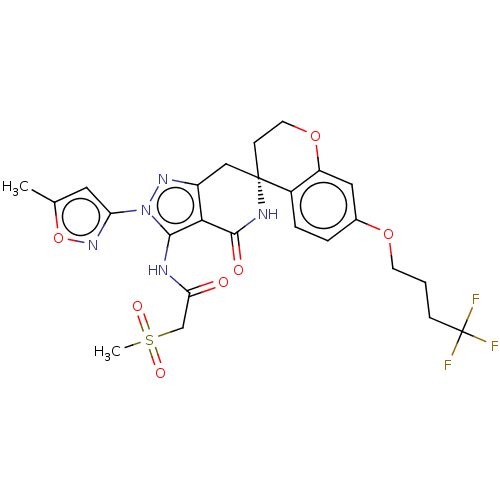
(US11198695, Example II-233)Show SMILES Cc1cc(no1)-n1nc2C[C@]3(CCOc4cc(OCCCC(F)(F)F)ccc34)NC(=O)c2c1NC(=O)CS(C)(=O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394211
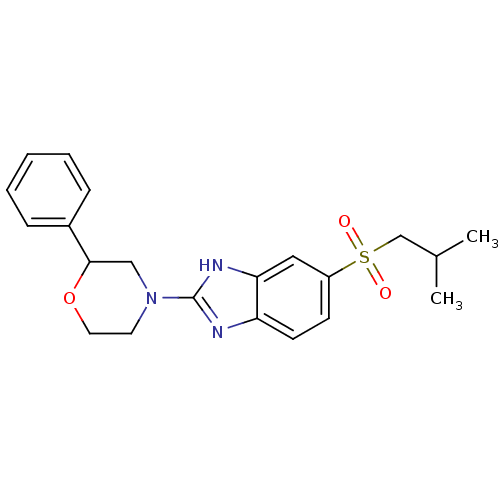
(CHEMBL2159165)Show SMILES CC(C)CS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C21H25N3O3S/c1-15(2)14-28(25,26)17-8-9-18-19(12-17)23-21(22-18)24-10-11-27-20(13-24)16-6-4-3-5-7-16/h3-9,12,15,20H,10-11,13-14H2,1-2H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529321
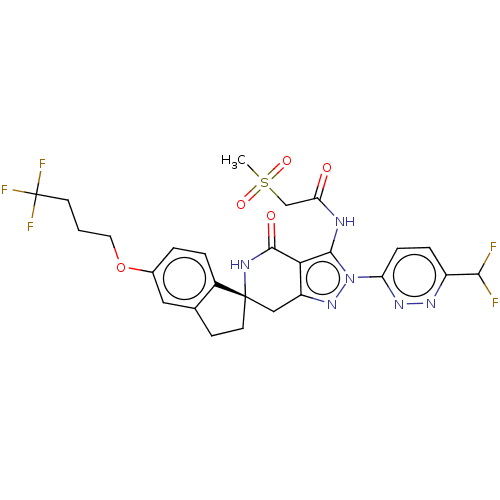
(US11198695, Example II-258)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCCCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(nn1)C(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390228
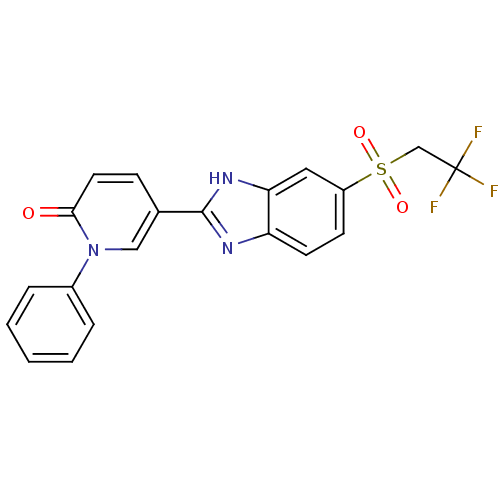
(CHEMBL2070161)Show SMILES FC(F)(F)CS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(=O)n(c1)-c1ccccc1 Show InChI InChI=1S/C20H14F3N3O3S/c21-20(22,23)12-30(28,29)15-7-8-16-17(10-15)25-19(24-16)13-6-9-18(27)26(11-13)14-4-2-1-3-5-14/h1-11H,12H2,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394221
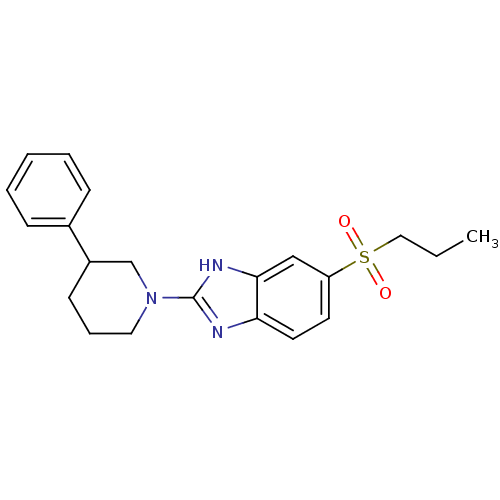
(CHEMBL2159158)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCCC(C1)c1ccccc1 Show InChI InChI=1S/C21H25N3O2S/c1-2-13-27(25,26)18-10-11-19-20(14-18)23-21(22-19)24-12-6-9-17(15-24)16-7-4-3-5-8-16/h3-5,7-8,10-11,14,17H,2,6,9,12-13,15H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390232
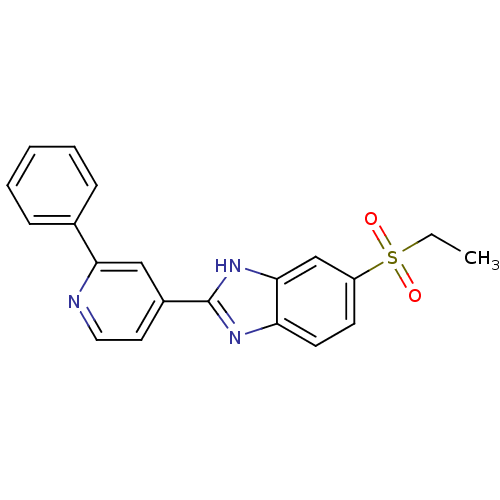
(CHEMBL2070157)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccnc(c1)-c1ccccc1 Show InChI InChI=1S/C20H17N3O2S/c1-2-26(24,25)16-8-9-17-19(13-16)23-20(22-17)15-10-11-21-18(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461644
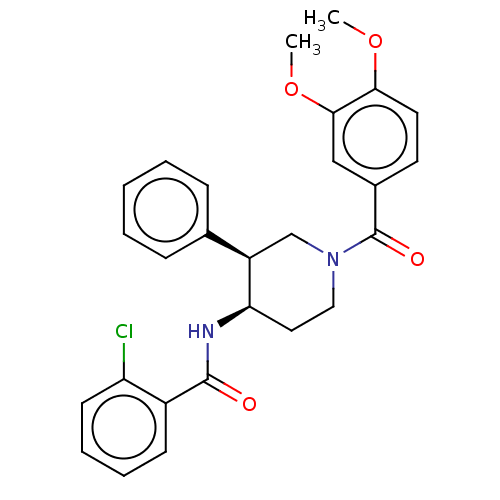
(CHEMBL4228416)Show SMILES COc1ccc(cc1OC)C(=O)N1CC[C@@H](NC(=O)c2ccccc2Cl)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C27H27ClN2O4/c1-33-24-13-12-19(16-25(24)34-2)27(32)30-15-14-23(21(17-30)18-8-4-3-5-9-18)29-26(31)20-10-6-7-11-22(20)28/h3-13,16,21,23H,14-15,17H2,1-2H3,(H,29,31)/t21-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529320
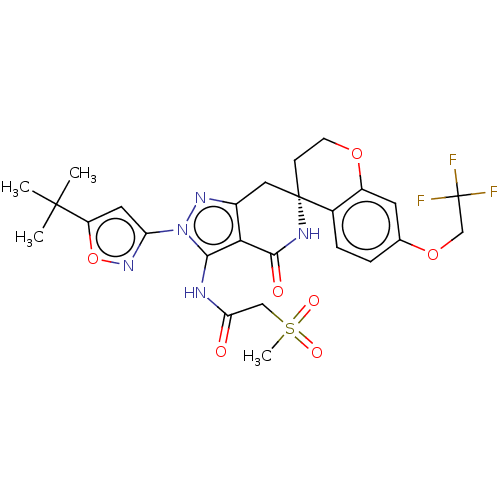
(US11198695, Example II-257)Show SMILES CC(C)(C)c1cc(no1)-n1nc2C[C@]3(CCOc4cc(OCC(F)(F)F)ccc34)NC(=O)c2c1NC(=O)CS(C)(=O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.409 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394214

(CHEMBL2159162)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H21N3O3S/c1-2-26(23,24)15-8-9-16-17(12-15)21-19(20-16)22-10-11-25-18(13-22)14-6-4-3-5-7-14/h3-9,12,18H,2,10-11,13H2,1H3,(H,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY-Y5 receptor |
Bioorg Med Chem Lett 23: 90-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.005
BindingDB Entry DOI: 10.7270/Q2FB547S |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394214

(CHEMBL2159162)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H21N3O3S/c1-2-26(23,24)15-8-9-16-17(12-15)21-19(20-16)22-10-11-25-18(13-22)14-6-4-3-5-7-14/h3-9,12,18H,2,10-11,13H2,1H3,(H,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390225

(CHEMBL2070140)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O2S/c1-2-13-27(25,26)19-11-12-20-21(15-19)24-22(23-20)18-10-6-9-17(14-18)16-7-4-3-5-8-16/h3-12,14-15H,2,13H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529322
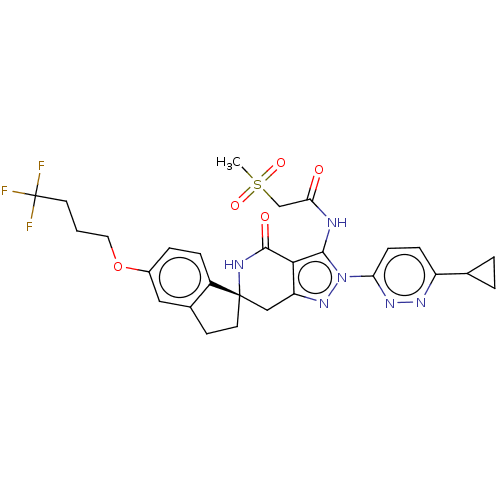
(US11198695, Example II-259)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCCCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(nn1)C1CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.439 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390222
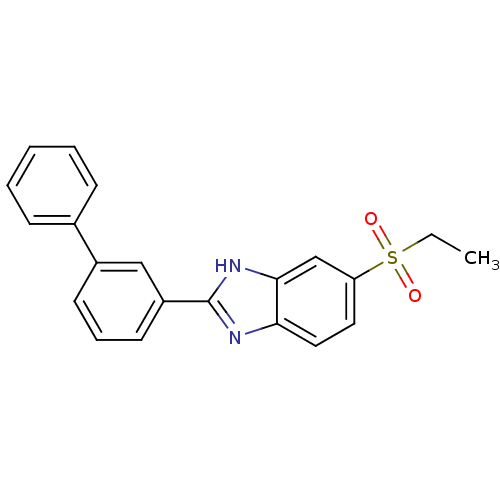
(CHEMBL2070137)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H18N2O2S/c1-2-26(24,25)18-11-12-19-20(14-18)23-21(22-19)17-10-6-9-16(13-17)15-7-4-3-5-8-15/h3-14H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461649
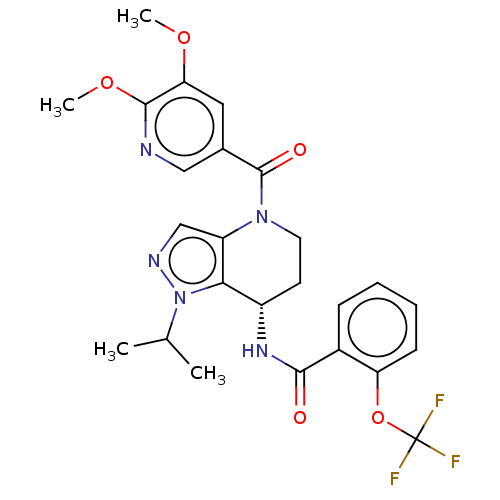
(CHEMBL4225519)Show SMILES COc1cc(cnc1OC)C(=O)N1CC[C@H](NC(=O)c2ccccc2OC(F)(F)F)c2c1cnn2C(C)C |r| Show InChI InChI=1S/C25H26F3N5O5/c1-14(2)33-21-17(31-22(34)16-7-5-6-8-19(16)38-25(26,27)28)9-10-32(18(21)13-30-33)24(35)15-11-20(36-3)23(37-4)29-12-15/h5-8,11-14,17H,9-10H2,1-4H3,(H,31,34)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390220
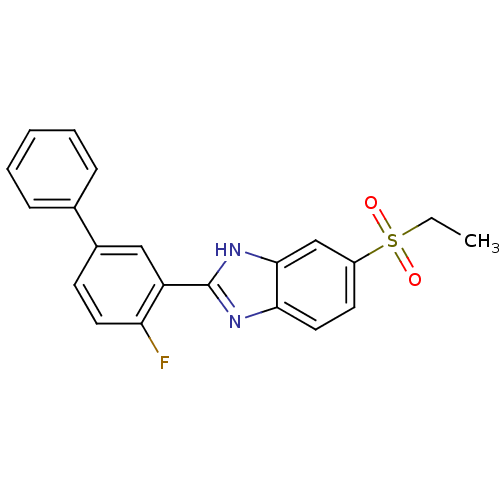
(CHEMBL2070147)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cc(ccc1F)-c1ccccc1 Show InChI InChI=1S/C21H17FN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)17-12-15(8-10-18(17)22)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390234

(CHEMBL2070155)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cc(ccn1)-c1ccccc1 Show InChI InChI=1S/C20H17N3O2S/c1-2-26(24,25)16-8-9-17-18(13-16)23-20(22-17)19-12-15(10-11-21-19)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394218

(CHEMBL2159156)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C20H23N3O3S/c1-2-12-27(24,25)16-8-9-17-18(13-16)22-20(21-17)23-10-11-26-19(14-23)15-6-4-3-5-7-15/h3-9,13,19H,2,10-12,14H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM403547
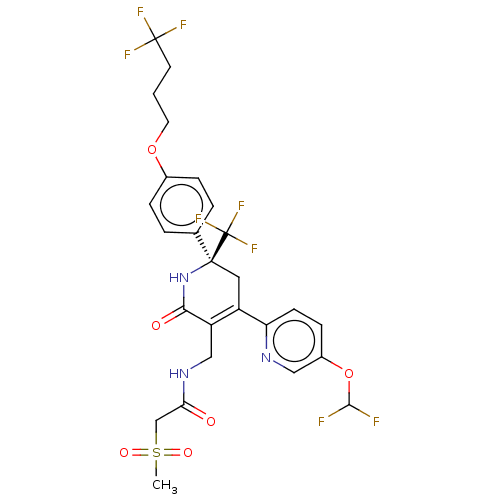
(US10335401, No. I-57)Show SMILES CS(=O)(=O)CC(=O)NCC1=C(C[C@](NC1=O)(c1ccc(OCCCC(F)(F)F)cc1)C(F)(F)F)c1ccc(OC(F)F)cn1 |r,t:9| Show InChI InChI=1S/C26H25F8N3O6S/c1-44(40,41)14-21(38)36-13-19-18(20-8-7-17(12-35-20)43-23(27)28)11-24(26(32,33)34,37-22(19)39)15-3-5-16(6-4-15)42-10-2-9-25(29,30)31/h3-8,12,23H,2,9-11,13-14H2,1H3,(H,36,38)(H,37,39)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
A full-length human MGAT2 gene to which a Flag-tag had been added at the N-terminal was inserted into pFastBac (from Invitrogen). A recombinant bacul... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2JM2D0H |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM528979

(US11198695, Example I-190 | US11198695, Example II...)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(cn1)C#N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.706 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461645
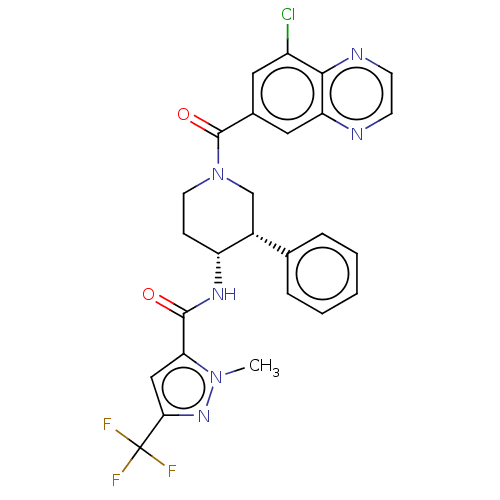
(CHEMBL4228472)Show SMILES Cn1nc(cc1C(=O)N[C@@H]1CCN(C[C@@H]1c1ccccc1)C(=O)c1cc(Cl)c2nccnc2c1)C(F)(F)F |r| Show InChI InChI=1S/C26H22ClF3N6O2/c1-35-21(13-22(34-35)26(28,29)30)24(37)33-19-7-10-36(14-17(19)15-5-3-2-4-6-15)25(38)16-11-18(27)23-20(12-16)31-8-9-32-23/h2-6,8-9,11-13,17,19H,7,10,14H2,1H3,(H,33,37)/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394223
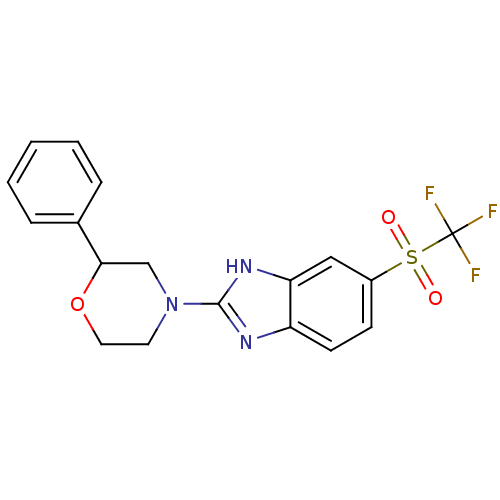
(CHEMBL2159166)Show SMILES FC(F)(F)S(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C18H16F3N3O3S/c19-18(20,21)28(25,26)13-6-7-14-15(10-13)23-17(22-14)24-8-9-27-16(11-24)12-4-2-1-3-5-12/h1-7,10,16H,8-9,11H2,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM403538
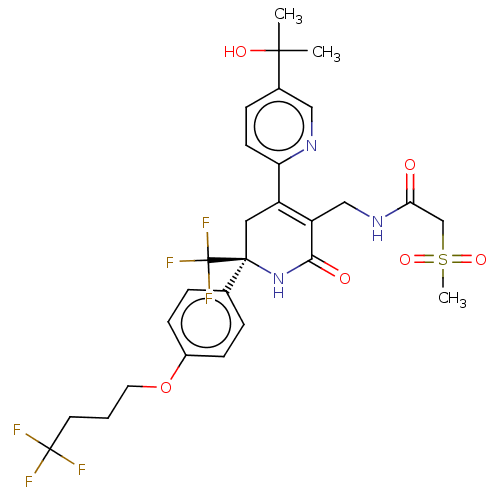
(US10335401, No. I-47)Show SMILES CC(C)(O)c1ccc(nc1)C1=C(CNC(=O)CS(C)(=O)=O)C(=O)N[C@@](C1)(c1ccc(OCCCC(F)(F)F)cc1)C(F)(F)F |r,c:11| Show InChI InChI=1S/C28H31F6N3O6S/c1-25(2,40)18-7-10-22(35-14-18)20-13-26(28(32,33)34,37-24(39)21(20)15-36-23(38)16-44(3,41)42)17-5-8-19(9-6-17)43-12-4-11-27(29,30)31/h5-10,14,40H,4,11-13,15-16H2,1-3H3,(H,36,38)(H,37,39)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
A full-length human MGAT2 gene to which a Flag-tag had been added at the N-terminal was inserted into pFastBac (from Invitrogen). A recombinant bacul... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2JM2D0H |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394228

(CHEMBL2159171)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccc(C)cc1 Show InChI InChI=1S/C20H23N3O3S/c1-3-27(24,25)16-8-9-17-18(12-16)22-20(21-17)23-10-11-26-19(13-23)15-6-4-14(2)5-7-15/h4-9,12,19H,3,10-11,13H2,1-2H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394227
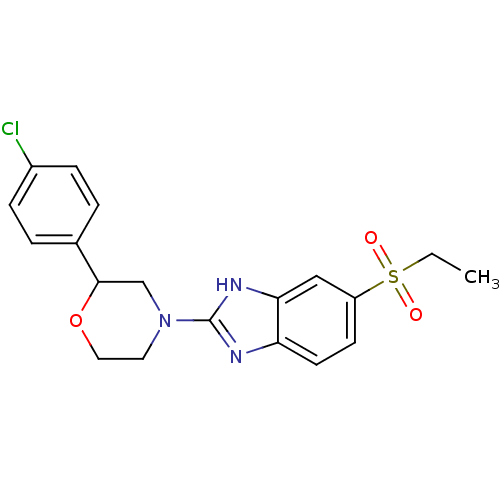
(CHEMBL2159170)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H20ClN3O3S/c1-2-27(24,25)15-7-8-16-17(11-15)22-19(21-16)23-9-10-26-18(12-23)13-3-5-14(20)6-4-13/h3-8,11,18H,2,9-10,12H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394470

(CHEMBL2160099)Show SMILES OCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-8-7-18-13-17(5-6-19(18)14-20)15-28-10-9-21(16-28)26-24(30)27-22-3-1-2-4-23(22)31-12-11-29/h1-8,13-14,21,29H,9-12,15-16H2,(H2,26,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor in human eosinophils assessed as inhibition of CCL11-induced degranulation after 4 hrs by ELISA |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297172
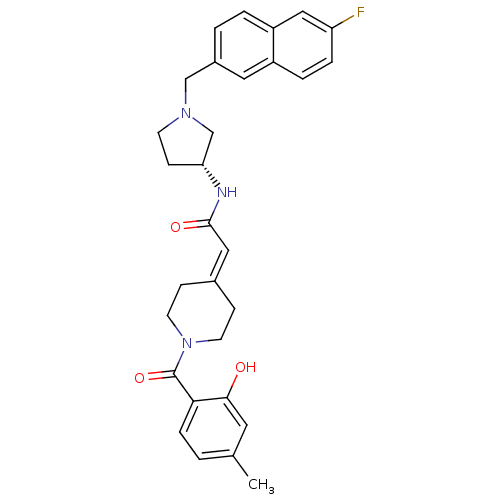
(CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-c1ccc(-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]/[#6](=O)-[#7]-[#6@@H]-2-[#6]-[#6]-[#7](-[#6]-c3ccc4cc(F)ccc4c3)-[#6]-2)c(-[#8])c1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-20-2-7-27(28(35)14-20)30(37)34-12-8-21(9-13-34)16-29(36)32-26-10-11-33(19-26)18-22-3-4-24-17-25(31)6-5-23(24)15-22/h2-7,14-17,26,35H,8-13,18-19H2,1H3,(H,32,36)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529360
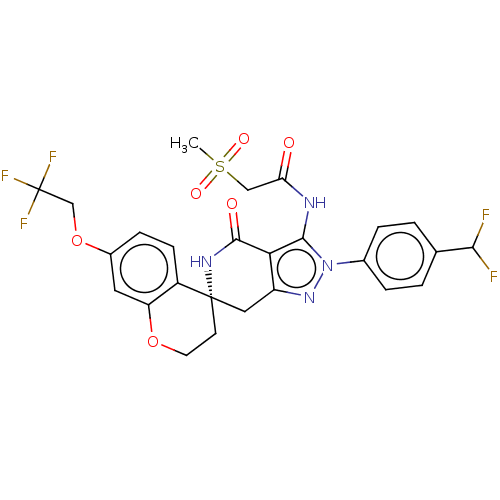
(US11198695, Example II-298)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCOc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(cc1)C(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529359
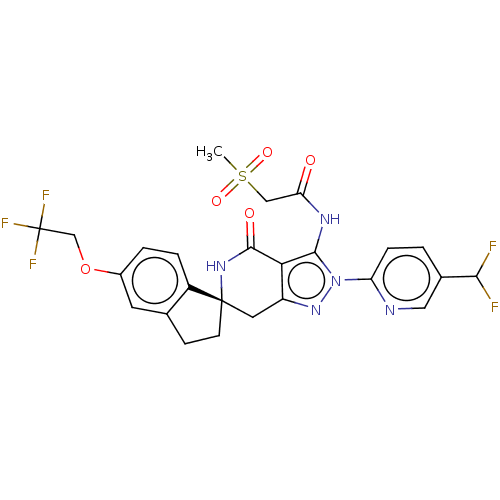
(US11198695, Example II-297)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(cn1)C(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529343
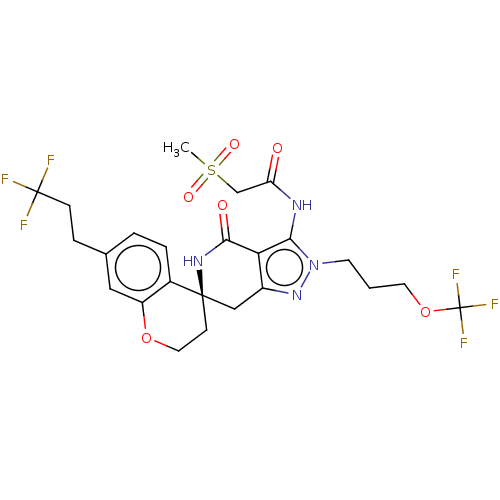
(US11198695, Example II-280)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@@]3(CCOc4cc(CCC(F)(F)F)ccc34)NC2=O)nn1CCCOC(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529344
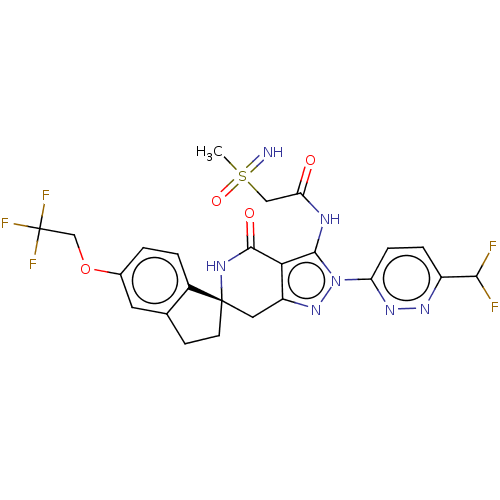
(US11198695, Example II-281)Show SMILES CS(=N)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(nn1)C(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529345
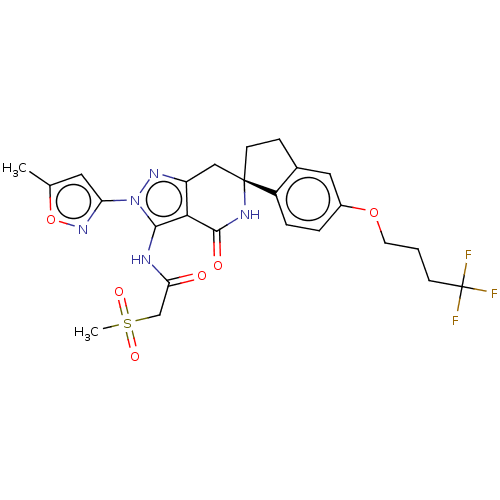
(US11198695, Example II-282)Show SMILES Cc1cc(no1)-n1nc2C[C@]3(CCc4cc(OCCCC(F)(F)F)ccc34)NC(=O)c2c1NC(=O)CS(C)(=O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529346
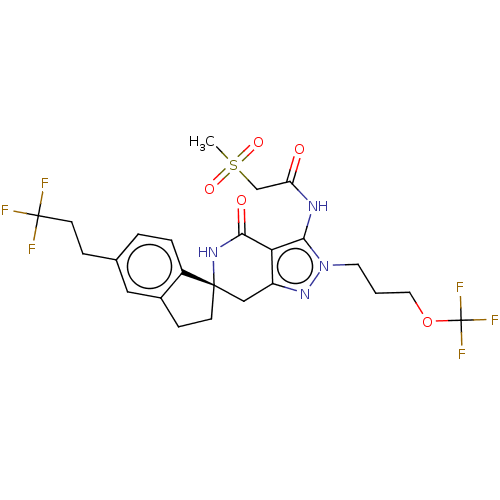
(US11198695, Example II-283)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(CCC(F)(F)F)ccc34)NC2=O)nn1CCCOC(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529347
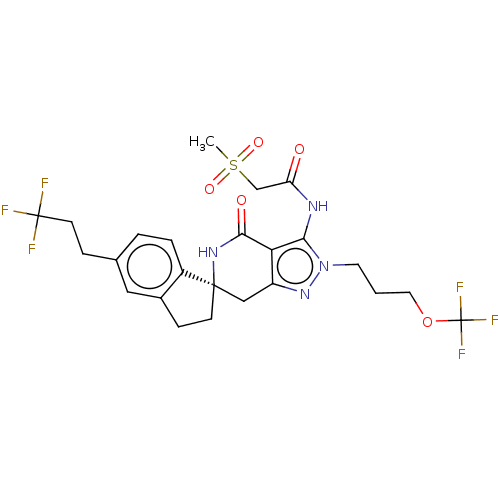
(US11198695, Example II-284)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@@]3(CCc4cc(CCC(F)(F)F)ccc34)NC2=O)nn1CCCOC(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529324
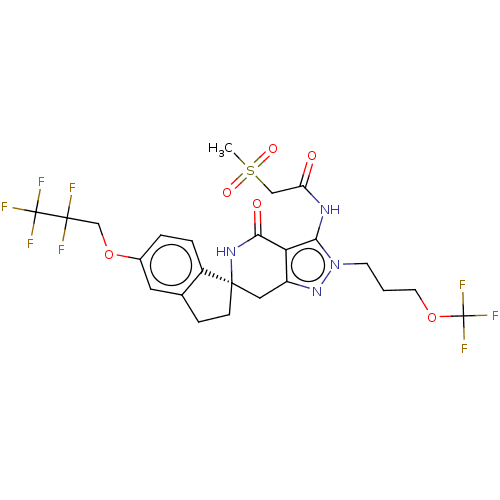
(US11198695, Example II-261)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@@]3(CCc4cc(OCC(F)(F)C(F)(F)F)ccc34)NC2=O)nn1CCCOC(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529358
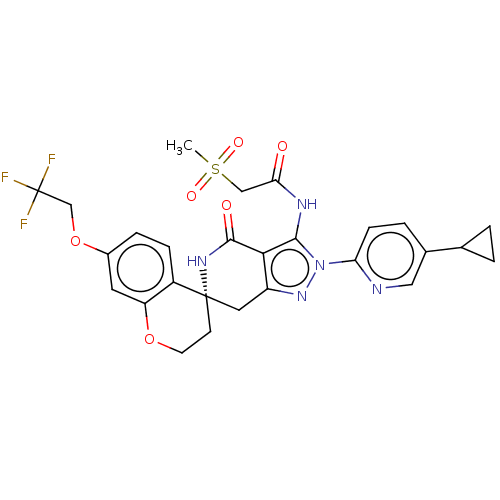
(US11198695, Example II-296)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCOc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(cn1)C1CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM528865
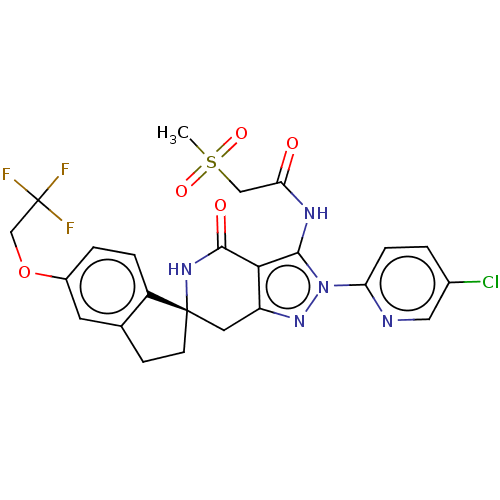
(US11198695, Example I-77)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCC(F)(F)F)ccc34)NC2=O)nn1-c1ccc(Cl)cn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529367
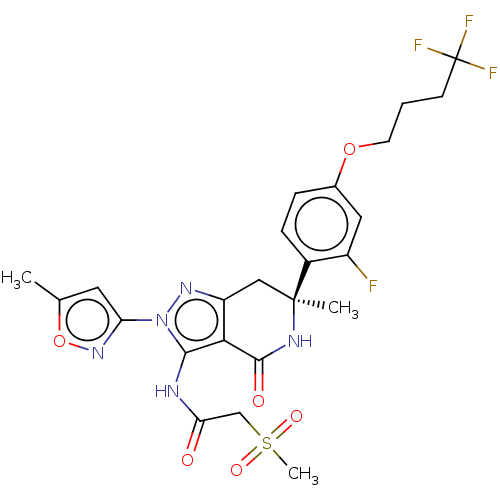
(US11198695, Example II-305)Show SMILES Cc1cc(no1)-n1nc2C[C@](C)(NC(=O)c2c1NC(=O)CS(C)(=O)=O)c1ccc(OCCCC(F)(F)F)cc1F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529353
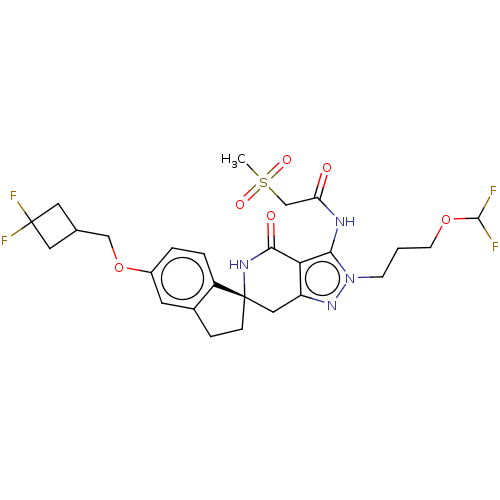
(US11198695, Example II-290)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@]3(CCc4cc(OCC5CC(F)(F)C5)ccc34)NC2=O)nn1CCCOC(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM529354
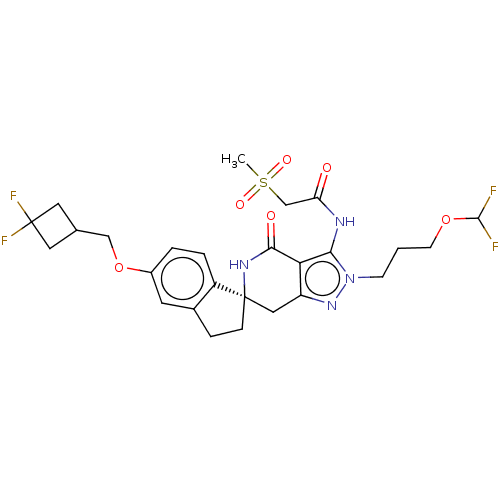
(US11198695, Example II-291)Show SMILES CS(=O)(=O)CC(=O)Nc1c2c(C[C@@]3(CCc4cc(OCC5CC(F)(F)C5)ccc34)NC2=O)nn1CCCOC(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21J9DXX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297171
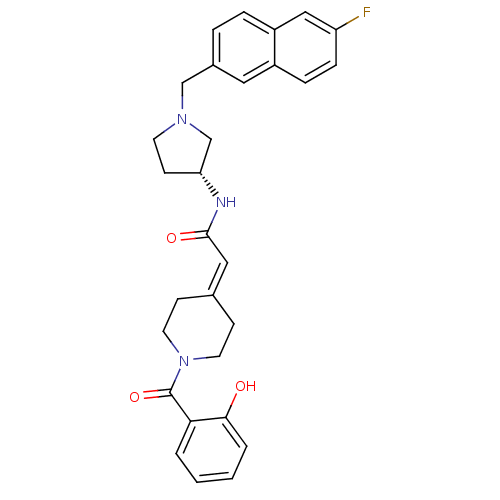
(CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-32-12-11-25(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)26-3-1-2-4-27(26)34/h1-8,15-17,25,34H,9-14,18-19H2,(H,31,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data