 Found 367 hits with Last Name = 'jiao' and Initial = 'y'
Found 367 hits with Last Name = 'jiao' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutathionylspermidine synthase
(Crithidia fasciculata) | BDBM50288580

((S)-4-Amino-4-[(S)-3-methyl-1-(phosphonomethyl-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)NCP(O)(O)=O Show InChI InChI=1S/C12H24N3O7P/c1-7(2)5-9(12(19)14-6-23(20,21)22)15-11(18)8(13)3-4-10(16)17/h7-9H,3-6,13H2,1-2H3,(H,14,19)(H,15,18)(H,16,17)(H2,20,21,22)/t8-,9-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for linear non-competitive inhibitory activity against glutathionylspermidine synthetase. |
Bioorg Med Chem Lett 6: 253-258 (1996)
Article DOI: 10.1016/0960-894X(96)00001-7
BindingDB Entry DOI: 10.7270/Q2T72HF8 |
More data for this
Ligand-Target Pair | |
Glutathionylspermidine synthase
(Crithidia fasciculata) | BDBM50288579

((S)-4-Amino-4-[(S)-2-methyl-1-(phosphonomethyl-car...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)NCP(O)(O)=O Show InChI InChI=1S/C11H22N3O7P/c1-6(2)9(11(18)13-5-22(19,20)21)14-10(17)7(12)3-4-8(15)16/h6-7,9H,3-5,12H2,1-2H3,(H,13,18)(H,14,17)(H,15,16)(H2,19,20,21)/t7-,9-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for linear non-competitive inhibitory activity against glutathionylspermidine synthetase. |
Bioorg Med Chem Lett 6: 253-258 (1996)
Article DOI: 10.1016/0960-894X(96)00001-7
BindingDB Entry DOI: 10.7270/Q2T72HF8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 |
J Med Chem 61: 140-157 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01091
BindingDB Entry DOI: 10.7270/Q2GB26GX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50385850

(CHEMBL2042853)Show SMILES Cc1cccnc1CN1CCC2(CC1)C(=O)N(c1ccccc21)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C31H29N3O/c1-23-8-7-19-32-28(23)22-33-20-17-31(18-21-33)27-11-5-6-12-29(27)34(30(31)35)26-15-13-25(14-16-26)24-9-3-2-4-10-24/h2-16,19H,17-18,20-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50251087

(CHEMBL4099365)Show SMILES COc1cc2ncnc(Oc3ccc(NC(=O)Nc4cnn(c4)C(C)C)c4ccccc34)c2cc1OC Show InChI InChI=1S/C27H26N6O4/c1-16(2)33-14-17(13-30-33)31-27(34)32-21-9-10-23(19-8-6-5-7-18(19)21)37-26-20-11-24(35-3)25(36-4)12-22(20)28-15-29-26/h5-16H,1-4H3,(H2,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using biotin substrate incubated for 1 hr by HTRF method |
J Med Chem 61: 140-157 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01091
BindingDB Entry DOI: 10.7270/Q2GB26GX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441
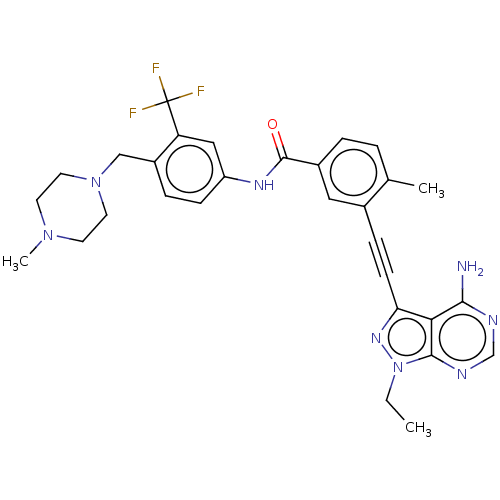
(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50251086

(CHEMBL4073263)Show SMILES COc1cc2ncnc(Oc3ccc(NC(=O)Nc4cc(C)on4)c4ccccc34)c2cc1OC Show InChI InChI=1S/C25H21N5O5/c1-14-10-23(30-35-14)29-25(31)28-18-8-9-20(16-7-5-4-6-15(16)18)34-24-17-11-21(32-2)22(33-3)12-19(17)26-13-27-24/h4-13H,1-3H3,(H2,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using biotin substrate incubated for 1 hr by HTRF method |
J Med Chem 61: 140-157 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01091
BindingDB Entry DOI: 10.7270/Q2GB26GX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
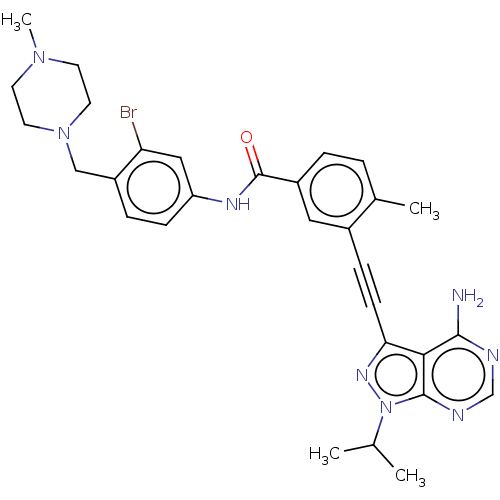
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50442444
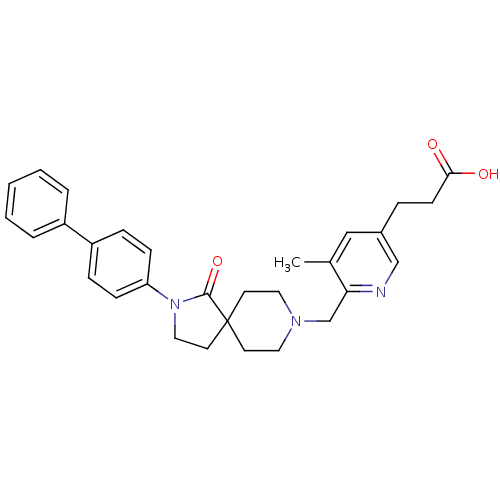
(CHEMBL2443222)Show SMILES Cc1cc(CCC(O)=O)cnc1CN1CCC2(CCN(C2=O)c2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C30H33N3O3/c1-22-19-23(7-12-28(34)35)20-31-27(22)21-32-16-13-30(14-17-32)15-18-33(29(30)36)26-10-8-25(9-11-26)24-5-3-2-4-6-24/h2-6,8-11,19-20H,7,12-18,21H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26041
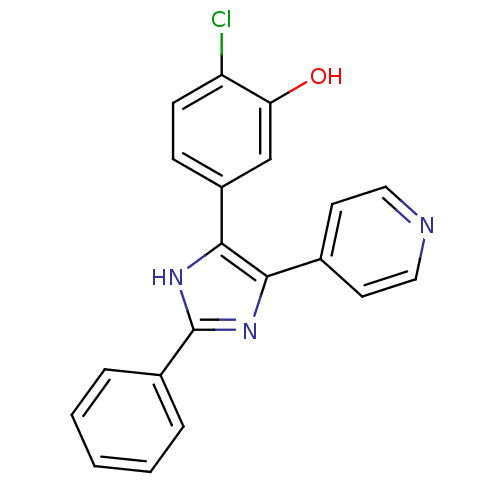
(2-chloro-5-(2-phenyl-5-(pyridin-4-yl)-1H-imidazol-...)Show SMILES Oc1cc(ccc1Cl)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C20H14ClN3O/c21-16-7-6-15(12-17(16)25)19-18(13-8-10-22-11-9-13)23-20(24-19)14-4-2-1-3-5-14/h1-12,25H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Raf1 kinase |
Bioorg Med Chem Lett 18: 2346-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.068
BindingDB Entry DOI: 10.7270/Q25X29S8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50251085

(CHEMBL4071203)Show SMILES COc1cc2ncnc(Oc3ccc(NC(=O)NC4CC4)c4ccccc34)c2cc1OC Show InChI InChI=1S/C24H22N4O4/c1-30-21-11-17-19(12-22(21)31-2)25-13-26-23(17)32-20-10-9-18(15-5-3-4-6-16(15)20)28-24(29)27-14-7-8-14/h3-6,9-14H,7-8H2,1-2H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using biotin substrate incubated for 1 hr by HTRF method |
J Med Chem 61: 140-157 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01091
BindingDB Entry DOI: 10.7270/Q2GB26GX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
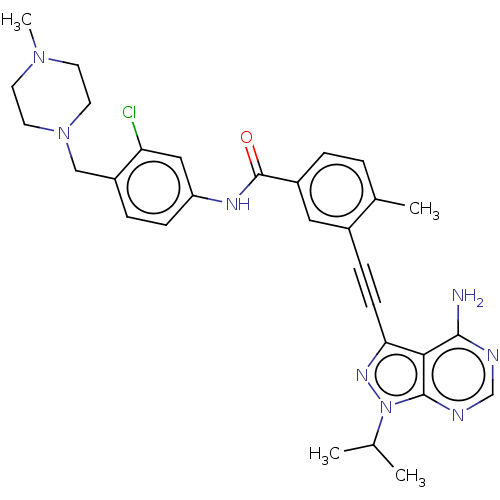
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
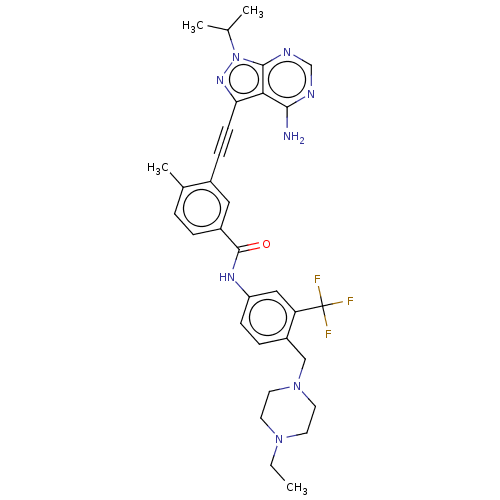
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
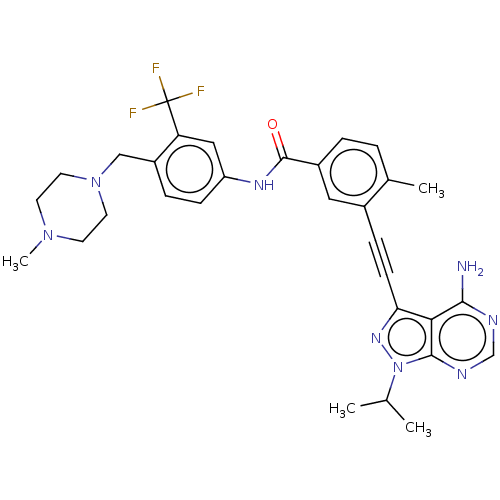
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50442445
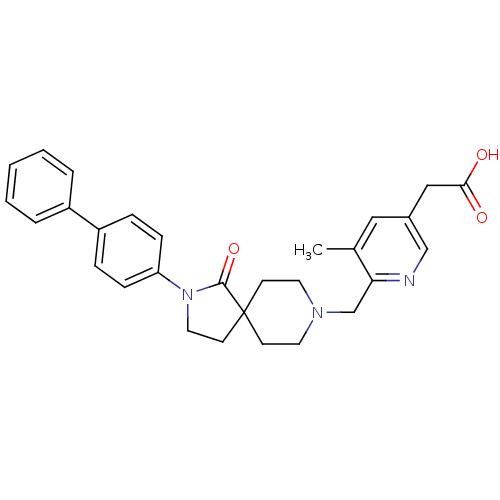
(CHEMBL2443221)Show SMILES Cc1cc(CC(O)=O)cnc1CN1CCC2(CCN(C2=O)c2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C29H31N3O3/c1-21-17-22(18-27(33)34)19-30-26(21)20-31-14-11-29(12-15-31)13-16-32(28(29)35)25-9-7-24(8-10-25)23-5-3-2-4-6-23/h2-10,17,19H,11-16,18,20H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL T315I mutant (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant Src using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50442461
(CHEMBL2443211)Show SMILES Cc1cccnc1CN1CCC2(CCN(C2=O)c2ccc(cc2C)-c2ccccc2)CC1 Show InChI InChI=1S/C28H31N3O/c1-21-7-6-15-29-25(21)20-30-16-12-28(13-17-30)14-18-31(27(28)32)26-11-10-24(19-22(26)2)23-8-4-3-5-9-23/h3-11,15,19H,12-14,16-18,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant RET (658 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331094
(4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)NC4CC4)c(Cl)c3)c2cc1C(N)=O Show InChI InChI=1S/C21H19ClN4O4/c1-29-19-10-17-13(9-14(19)20(23)27)18(6-7-24-17)30-12-4-5-16(15(22)8-12)26-21(28)25-11-2-3-11/h4-11H,2-3H2,1H3,(H2,23,27)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 |
J Med Chem 61: 140-157 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01091
BindingDB Entry DOI: 10.7270/Q2GB26GX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50568997
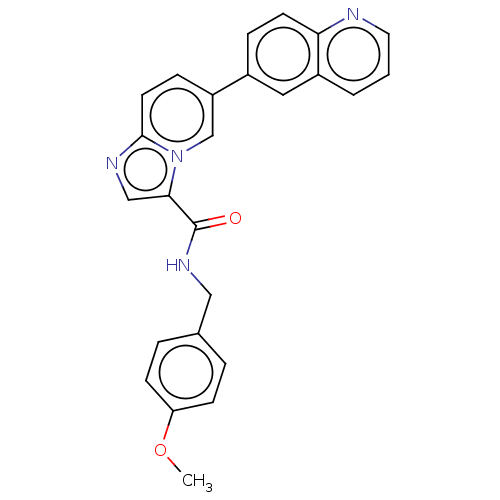
(CHEMBL4853178)Show SMILES COc1ccc(CNC(=O)c2cnc3ccc(cn23)-c2ccc3ncccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50442458

(CHEMBL2443114)Show SMILES Cc1cccnc1CN1CCC2(CCN(C2=O)c2ccc(cc2)-c2ccc(cc2)C(O)=O)CC1 Show InChI InChI=1S/C28H29N3O3/c1-20-3-2-15-29-25(20)19-30-16-12-28(13-17-30)14-18-31(27(28)34)24-10-8-22(9-11-24)21-4-6-23(7-5-21)26(32)33/h2-11,15H,12-14,16-19H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50569002
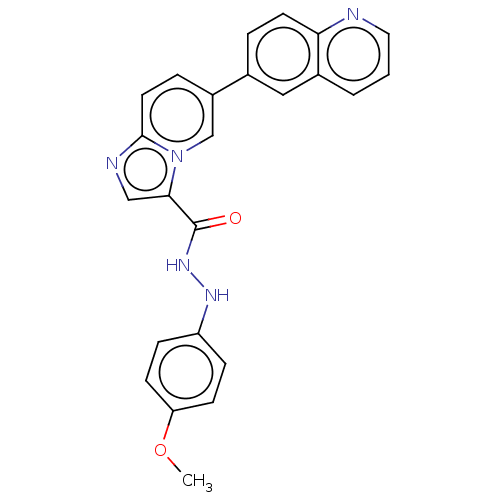
(CHEMBL4861035)Show SMILES COc1ccc(NNC(=O)c2cnc3ccc(cn23)-c2ccc3ncccc3c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Fyn |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50375259

(CHEMBL220019)Show SMILES CNC(=O)Oc1cc(C)c(C=Cc2cncc(c2)C(O)=O)c(C)c1 |w:11.11| Show InChI InChI=1S/C18H18N2O4/c1-11-6-15(24-18(23)19-3)7-12(2)16(11)5-4-13-8-14(17(21)22)10-20-9-13/h4-10H,1-3H3,(H,19,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Raf1 kinase |
Bioorg Med Chem Lett 18: 2346-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.068
BindingDB Entry DOI: 10.7270/Q25X29S8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50139601
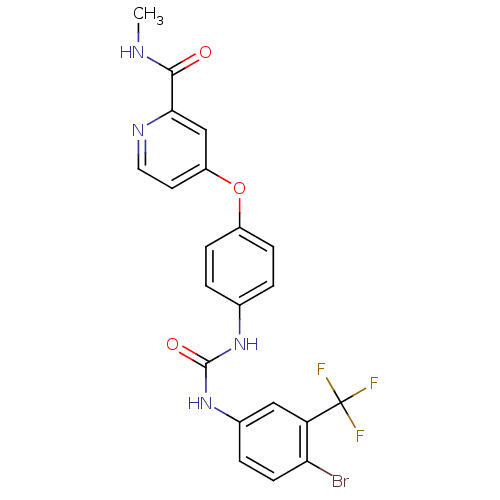
(1-(4-bromo-3-(trifluoromethyl)phenyl)-3-(4-(2-(met...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Br)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16BrF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Raf1 kinase |
Bioorg Med Chem Lett 18: 2346-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.068
BindingDB Entry DOI: 10.7270/Q25X29S8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50568986

(CHEMBL4853617)Show SMILES COc1ccc(CNC(=O)c2cnc3ccc(cn23)-c2cnn(C)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant TXK (256 to end residues) using GEEPLYWSFPAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50569001
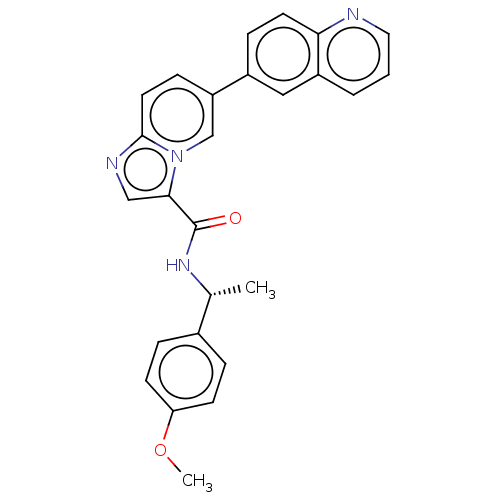
(CHEMBL4878682)Show SMILES COc1ccc(cc1)[C@@H](C)NC(=O)c1cnc2ccc(cn12)-c1ccc2ncccc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant FYN using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50568999
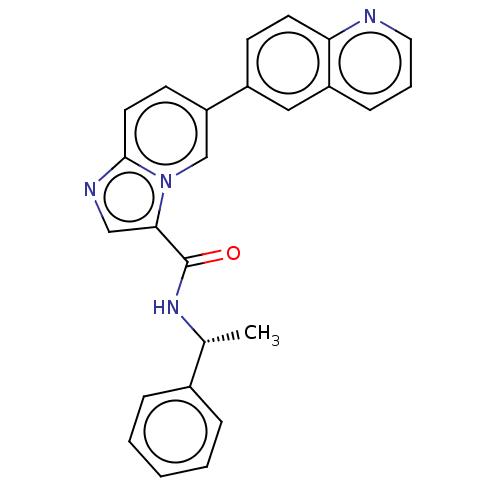
(CHEMBL4878376)Show SMILES C[C@@H](NC(=O)c1cnc2ccc(cn12)-c1ccc2ncccc2c1)c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50375258
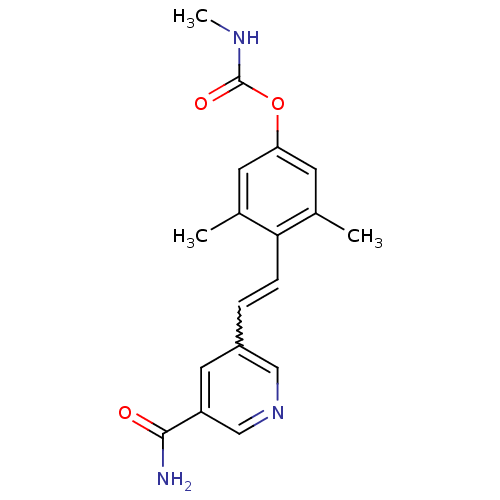
(CHEMBL219870)Show SMILES CNC(=O)Oc1cc(C)c(C=Cc2cncc(c2)C(N)=O)c(C)c1 |w:11.11| Show InChI InChI=1S/C18H19N3O3/c1-11-6-15(24-18(23)20-3)7-12(2)16(11)5-4-13-8-14(17(19)22)10-21-9-13/h4-10H,1-3H3,(H2,19,22)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Raf1 kinase |
Bioorg Med Chem Lett 18: 2346-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.068
BindingDB Entry DOI: 10.7270/Q25X29S8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086445

(CHEMBL3426229 | US10266537, Compound 21)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086444

(CHEMBL3426230 | US10266537, Compound 20)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FGFR1 (456 to 765 residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086449

(CHEMBL3426224 | US10266537, Compound 8)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H29F3N8O/c1-18-4-5-20(14-19(18)7-9-24-25-26(33)34-17-35-27(25)39(3)37-24)28(41)36-22-8-6-21(23(15-22)29(30,31)32)16-40-12-10-38(2)11-13-40/h4-6,8,14-15,17H,10-13,16H2,1-3H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50568991
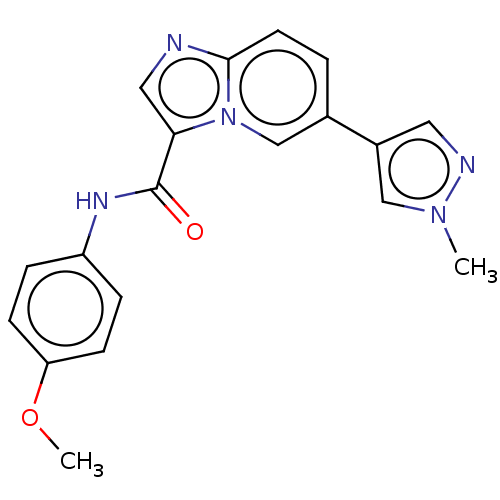
(CHEMBL4871915)Show SMILES COc1ccc(NC(=O)c2cnc3ccc(cn23)-c2cnn(C)c2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVR as substrate incubated for 40 mins in presence of [gamma-33ATP] by sci... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127881
BindingDB Entry DOI: 10.7270/Q2HX1HDJ |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50442463

(CHEMBL2443129)Show SMILES COc1cc(ccc1N1CCC2(CCN(Cc3ncccc3C)CC2)C1=O)-c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-21-7-6-15-29-24(21)20-30-16-12-28(13-17-30)14-18-31(27(28)32)25-11-10-23(19-26(25)33-2)22-8-4-3-5-9-22/h3-11,15,19H,12-14,16-18,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PHD2 (unknown origin) by HTRF assay |
Bioorg Med Chem 21: 6349-58 (2013)
Article DOI: 10.1016/j.bmc.2013.08.046
BindingDB Entry DOI: 10.7270/Q2K075Q1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data