

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50370683 (CHEMBL1169543) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50384537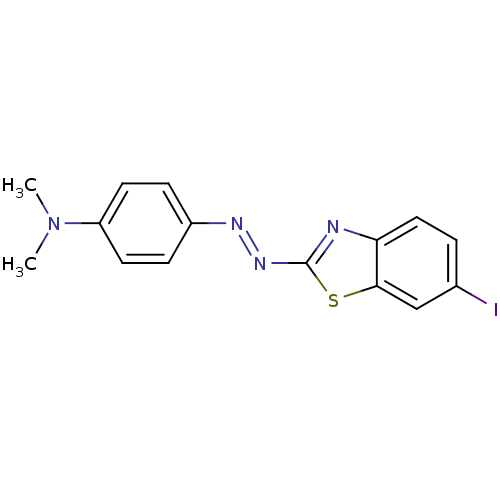 (CHEMBL2036430) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay | ACS Med Chem Lett 3: 58-62 (2012) Article DOI: 10.1021/ml200230e BindingDB Entry DOI: 10.7270/Q2PR7X1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50370683 (CHEMBL1169543) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50492526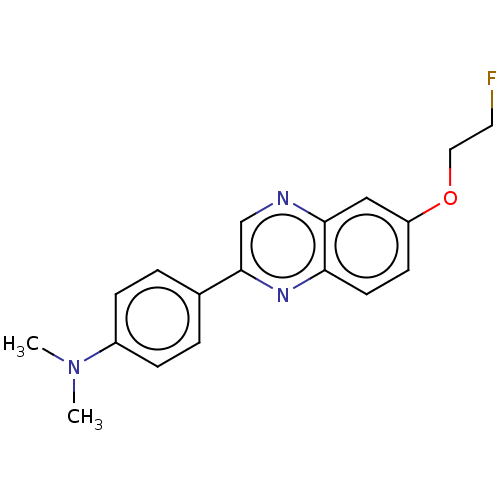 (CHEMBL2407616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50492522 (CHEMBL2407622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175574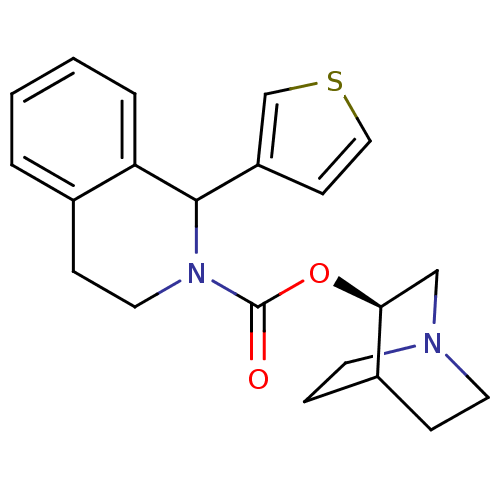 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175570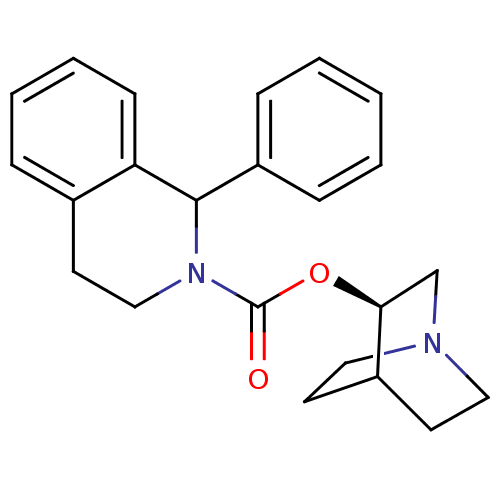 ((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175581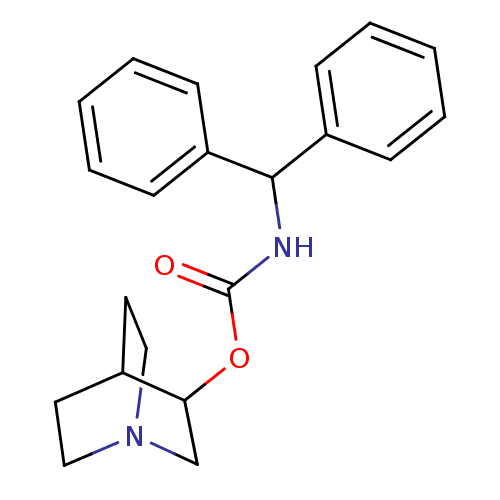 (Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175575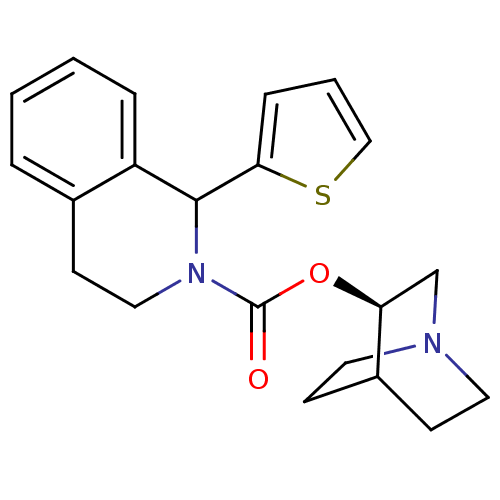 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175575 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175581 (Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175574 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175575 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175575 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175578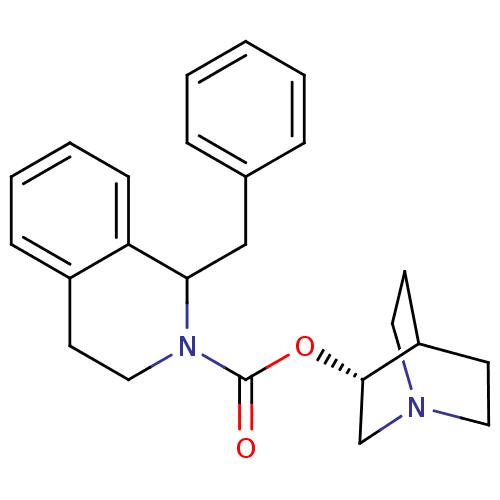 (1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175578 (1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165019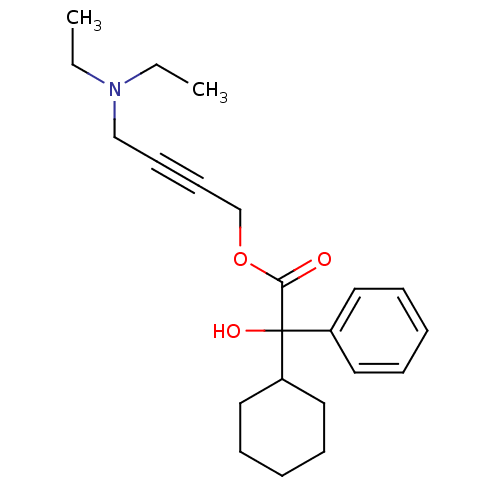 (4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175570 ((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50165019 (4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175566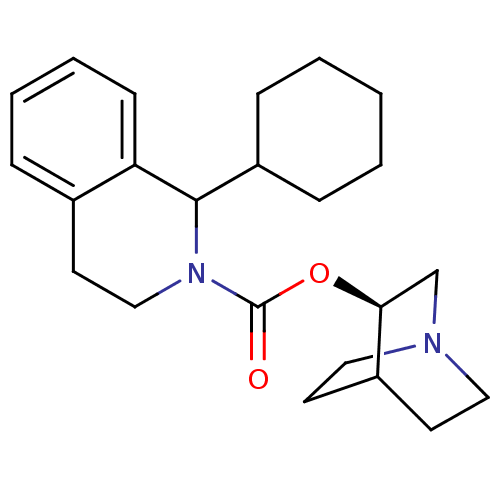 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175566 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175566 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175566 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175581 (Benzhydryl-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50122787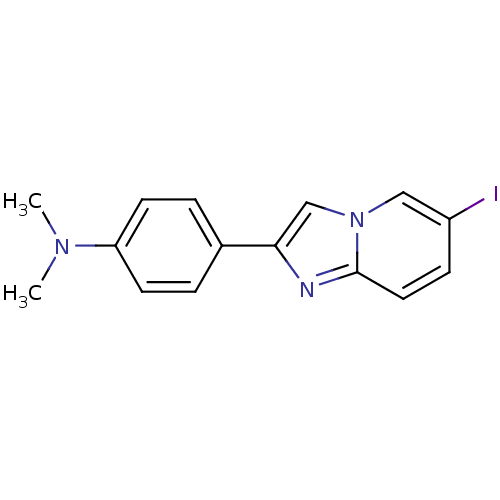 (2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175574 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175567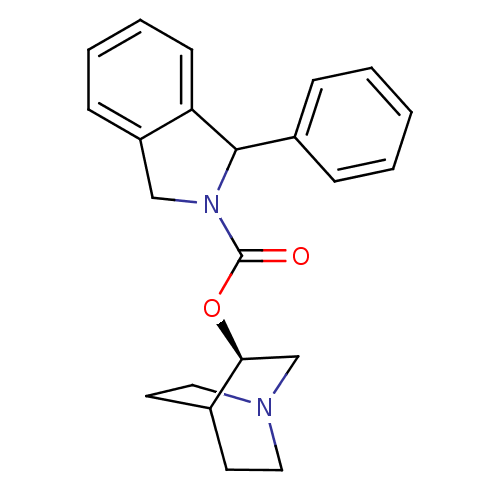 (1-Phenyl-1,3-dihydro-isoindole-2-carboxylic acid (...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50370683 (CHEMBL1169543) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175569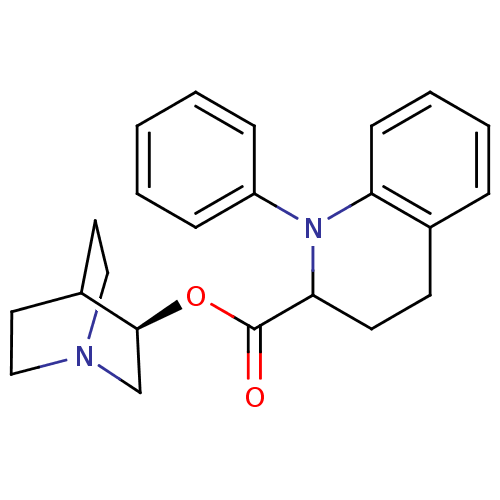 (1-Phenyl-1,2,3,4-tetrahydro-quinoline-2-carboxylic...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50370682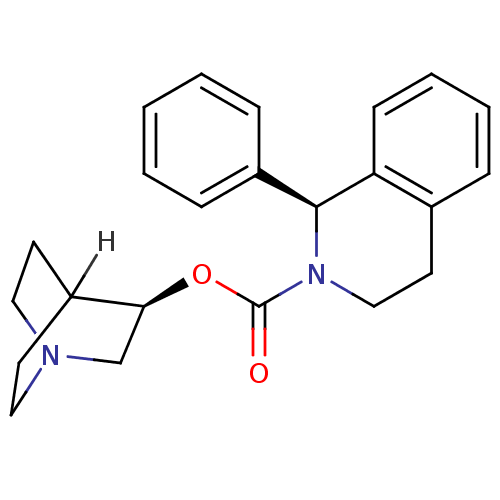 (SOLIFENACIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50370682 (SOLIFENACIN) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50175572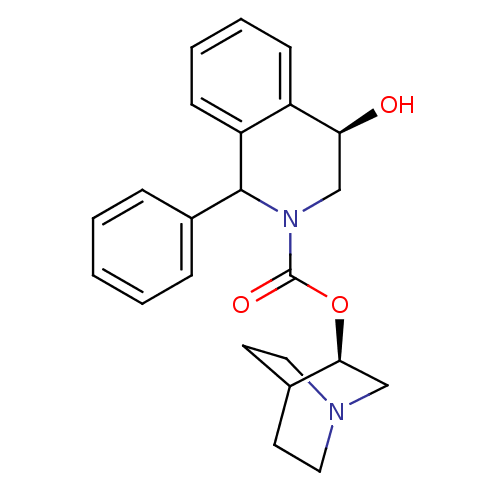 ((2R,4R)-4-Hydroxy-1-phenyl-3,4-dihydro-1H-isoquino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat cortex muscarinic acetylcholine receptor M1 using [3H]pirenzepine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50492518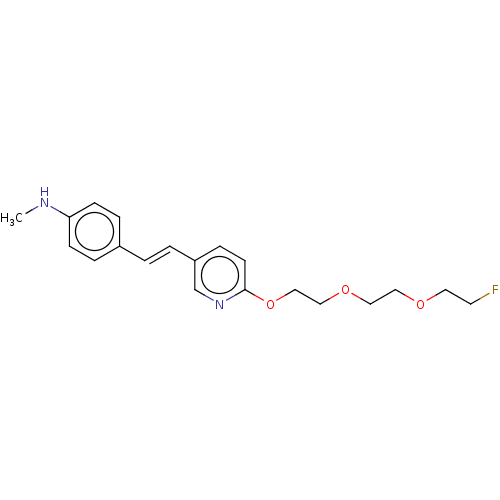 (Florbetapir | US10906900, AV45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50384538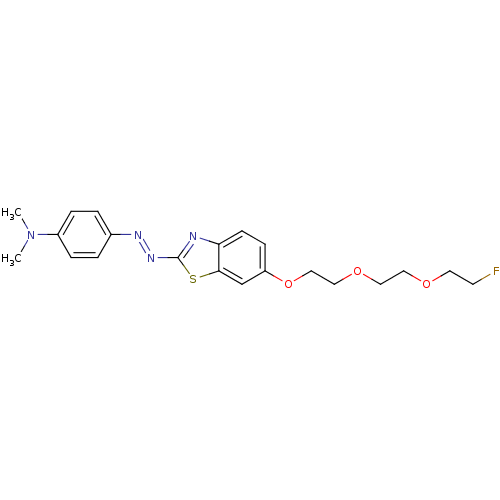 (CHEMBL2036419 | CHEMBL2036420) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of Thioflavin S from recombinant human tau expressed in Escherichia coli after 30 mins by fluorescence assay | ACS Med Chem Lett 3: 58-62 (2012) Article DOI: 10.1021/ml200230e BindingDB Entry DOI: 10.7270/Q2PR7X1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175575 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175575 (1-Thiophen-2-yl-3,4-dihydro-1H-isoquinoline-2-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50165019 (4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175574 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50492519 (CHEMBL2407615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]IMPY from amyloid beta (1 to 42) (unknown origin) after 3 hrs by gamma counting | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50492516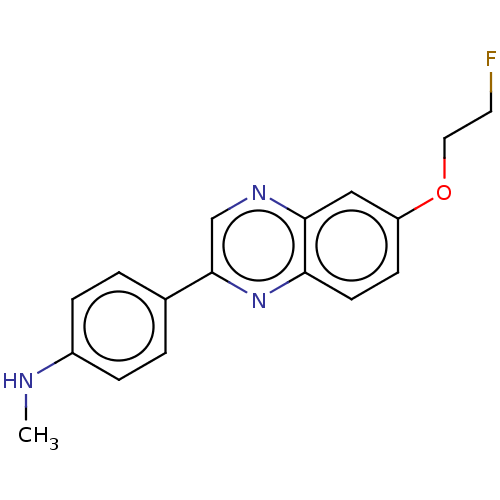 (CHEMBL2407621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to beta-amyloid plaque in Alzheimer's disease patient brain cortex after 1 hr by thioflavin-S based autoradiography | ACS Med Chem Lett 4: 596-600 (2013) Article DOI: 10.1021/ml4000707 BindingDB Entry DOI: 10.7270/Q2RX9G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein tau (Homo sapiens (Human)) | BDBM50390102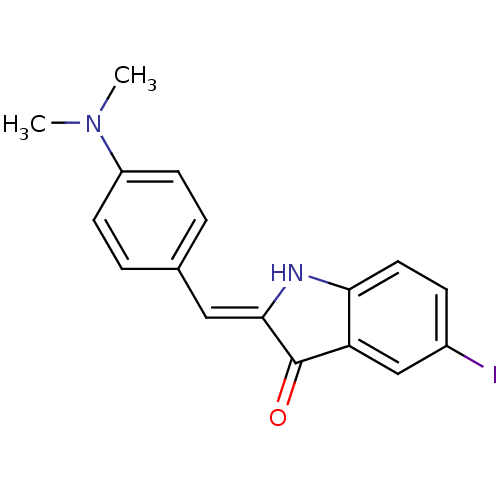 (CHEMBL2069433) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to human Tau aggregates after 30 mins by Thioflavin-S-based fluorescence assay | Bioorg Med Chem Lett 22: 5700-3 (2012) Article DOI: 10.1016/j.bmcl.2012.06.086 BindingDB Entry DOI: 10.7270/Q2TQ62K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175574 ((S)-1-Thiophen-3-yl-3,4-dihydro-1H-isoquinoline-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175566 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175566 (1-Cyclohexyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175578 (1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175578 (1-Benzyl-3,4-dihydro-1H-isoquinoline-2-carboxylic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175569 (1-Phenyl-1,2,3,4-tetrahydro-quinoline-2-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175567 (1-Phenyl-1,3-dihydro-isoindole-2-carboxylic acid (...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50175570 ((S)-1-Phenyl-3,4-dihydro-1H-isoquinoline-2-carboxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat heart muscarinic acetylcholine receptor M2 using [3H]quinuclidinyl benzilate | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50175572 ((2R,4R)-4-Hydroxy-1-phenyl-3,4-dihydro-1H-isoquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Binding affinity for rat salivary gland muscarinic acetylcholine receptor M3 using [3H]N-methylscopolamine | J Med Chem 48: 6597-606 (2005) Article DOI: 10.1021/jm050099q BindingDB Entry DOI: 10.7270/Q2NP257C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 268 total ) | Next | Last >> |