

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240305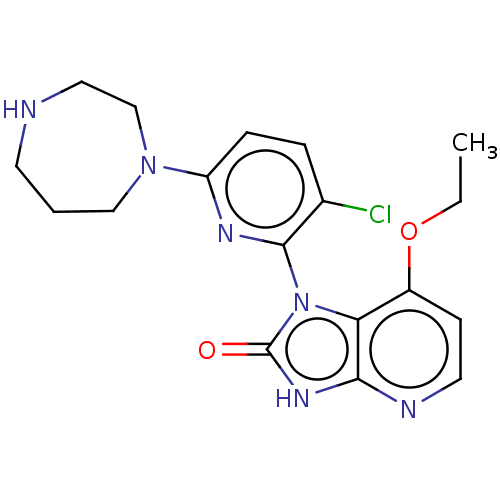 (CHEMBL4082370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240307 (CHEMBL4065996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240298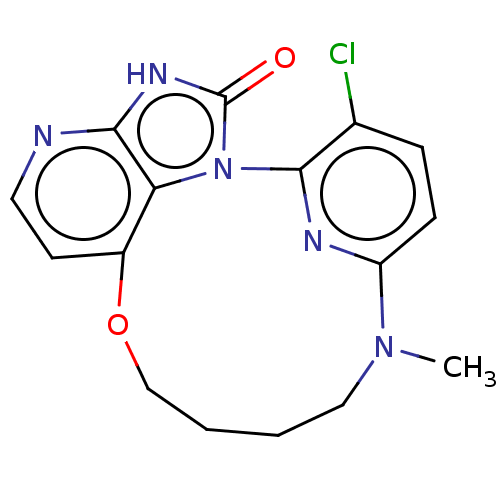 (CHEMBL4092652) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260529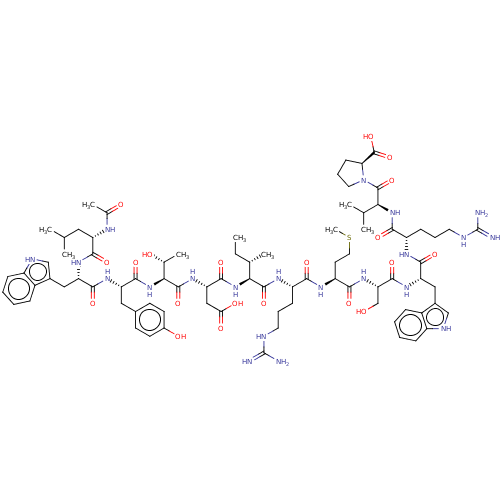 (CHEMBL4077626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of C-terminal biotin-labelled BCoR (Arg498 to 514Pro residues) binding to recombinant FLAG-tagged BCL6 BTB (5 to 129 residues) (unknown or... | Bioorg Med Chem 25: 4876-4886 (2017) Article DOI: 10.1016/j.bmc.2017.07.037 BindingDB Entry DOI: 10.7270/Q2G44SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240297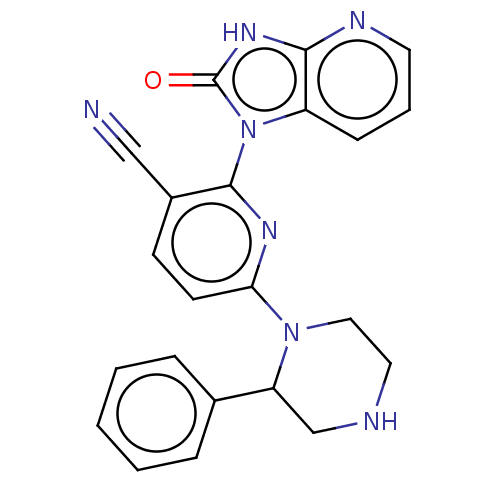 (CHEMBL4100381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240303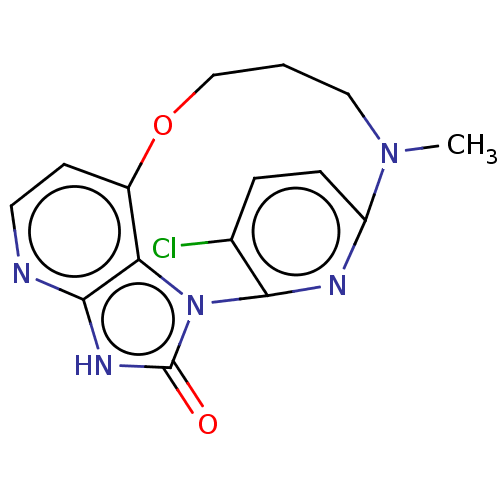 (CHEMBL4093591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240304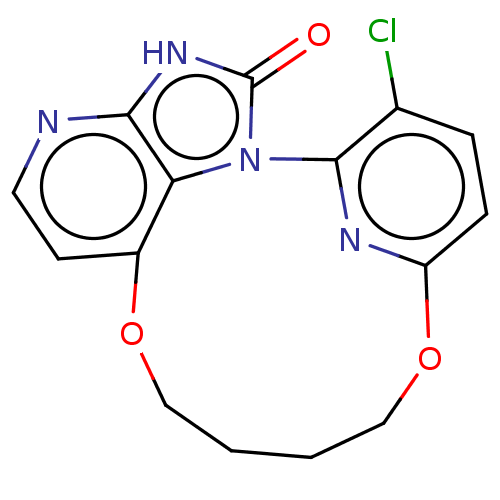 (CHEMBL4099368) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240301 (CHEMBL4074629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240302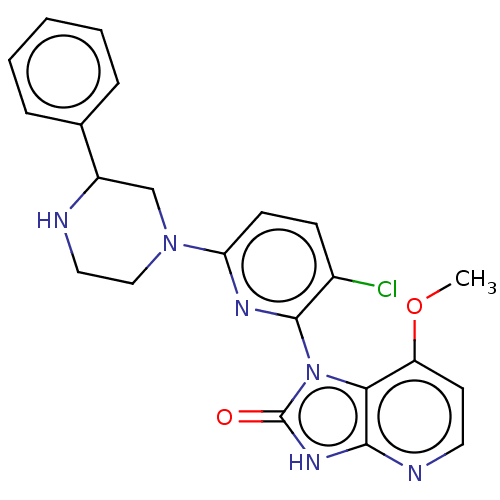 (CHEMBL4101327) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240299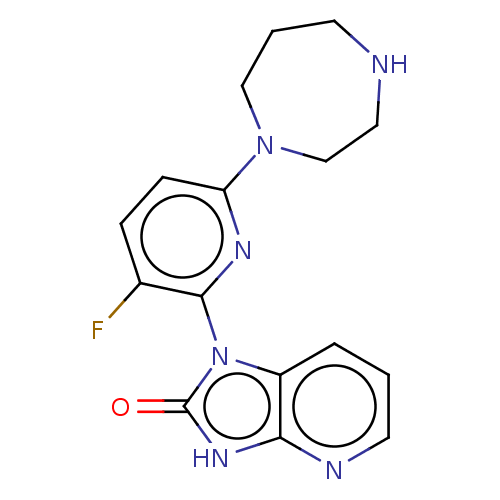 (CHEMBL4071874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240300 (CHEMBL4063243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528572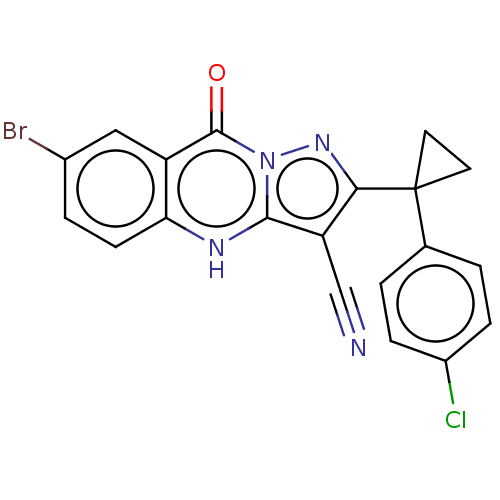 (US11192893, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528573 (US11192893, Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528576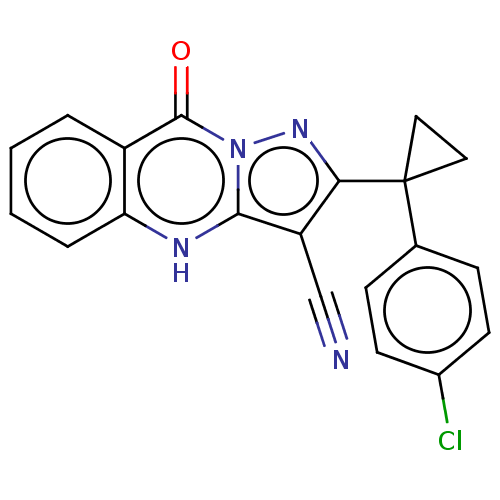 (US11192893, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528579 (US11192893, Example 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50240306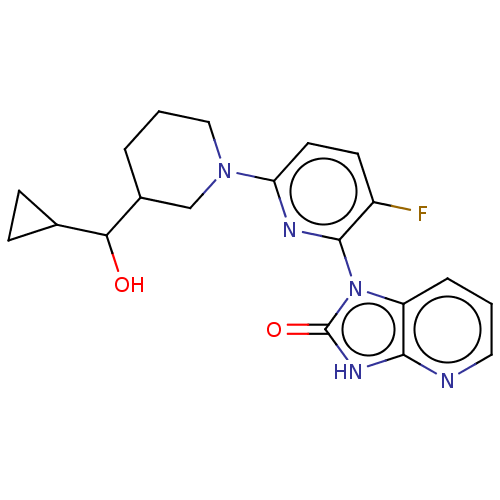 (CHEMBL4084895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged PKCtheta (unknown origin) expressed in baculovirus expression system using fluorescein-PKC as substrate preincub... | Bioorg Med Chem Lett 27: 2497-2501 (2017) Article DOI: 10.1016/j.bmcl.2017.03.099 BindingDB Entry DOI: 10.7270/Q21G0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528571 (US11192893, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dynein light chain 2, cytoplasmic (Human) | BDBM528570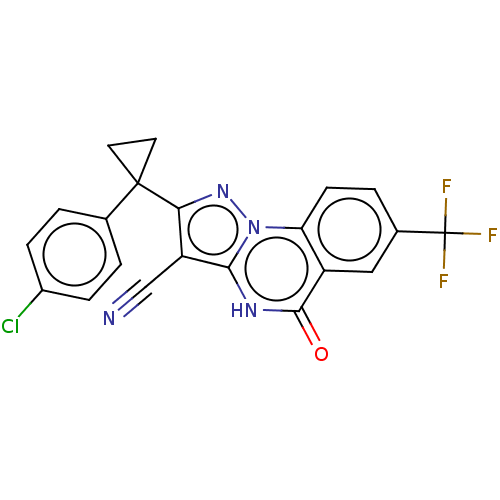 (US11192893, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested employing a microtubule gliding assay, a standard biochemical assay for motor proteins. The first set of studies focused on hum... | Citation and Details BindingDB Entry DOI: 10.7270/Q22J6G18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50561244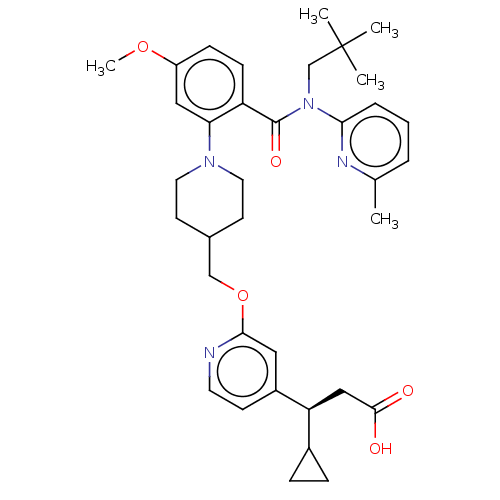 (CHEMBL4794409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260534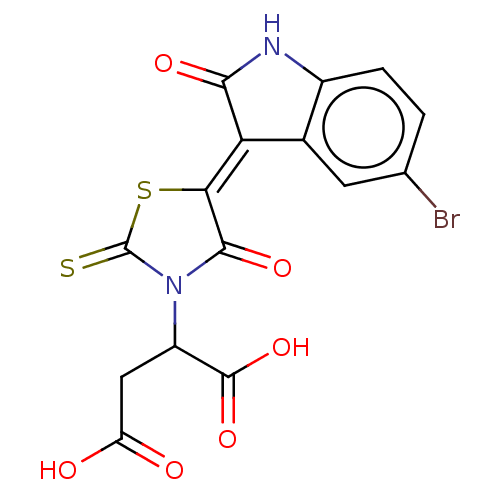 (CHEMBL4066375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of C-terminal biotin-labelled BCoR/wild-type BCL6 BTB domain (unknown origin) protein-protein interaction by ELISA | Bioorg Med Chem 25: 4876-4886 (2017) Article DOI: 10.1016/j.bmc.2017.07.037 BindingDB Entry DOI: 10.7270/Q2G44SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561243 (CHEMBL4792979) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561242 (CHEMBL4748702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561245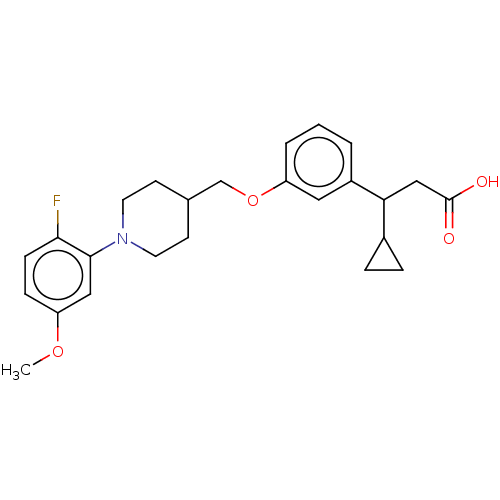 (CHEMBL4757231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561246 (CHEMBL4784293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561247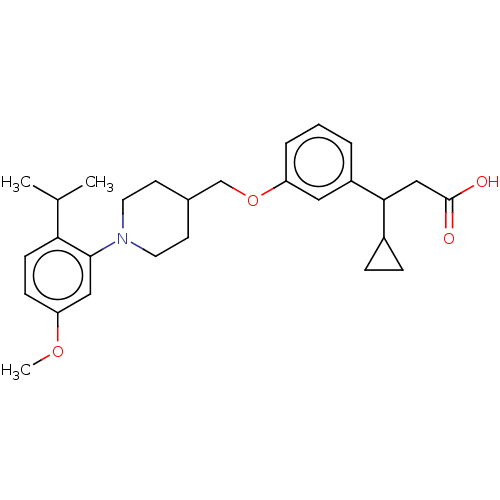 (CHEMBL4760668) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561248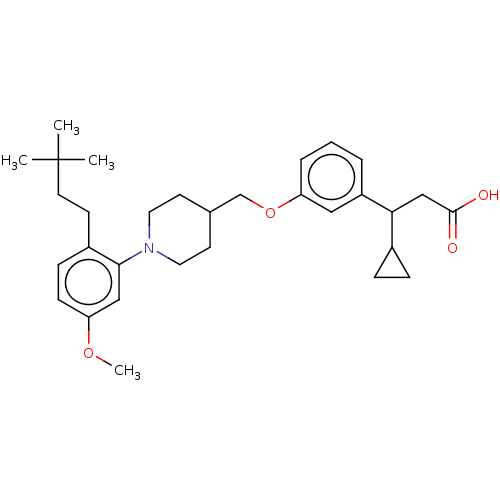 (CHEMBL4764150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561249 (CHEMBL4755717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561250 (CHEMBL4754718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561251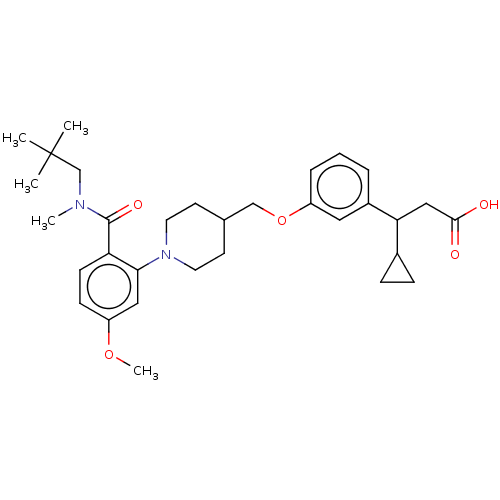 (CHEMBL4746614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561252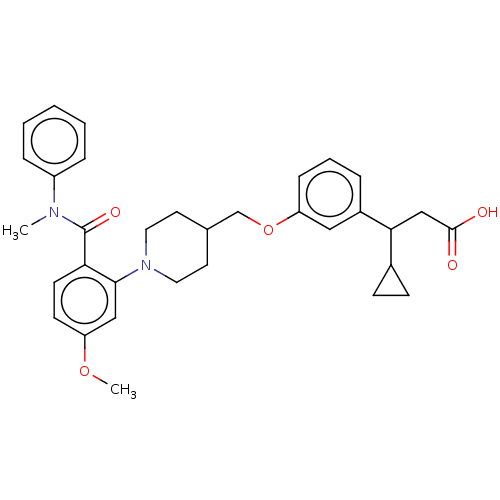 (CHEMBL4789994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561253 (CHEMBL4786992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561254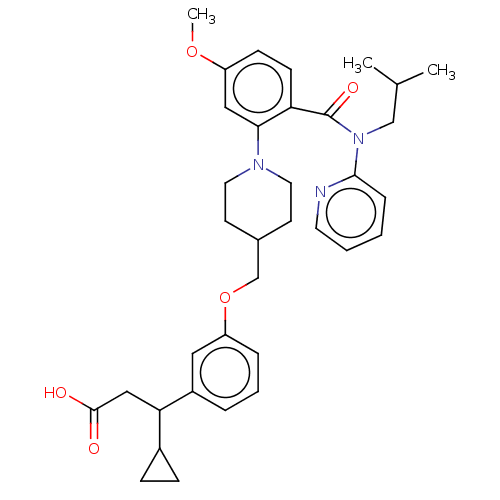 (CHEMBL4748739) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561255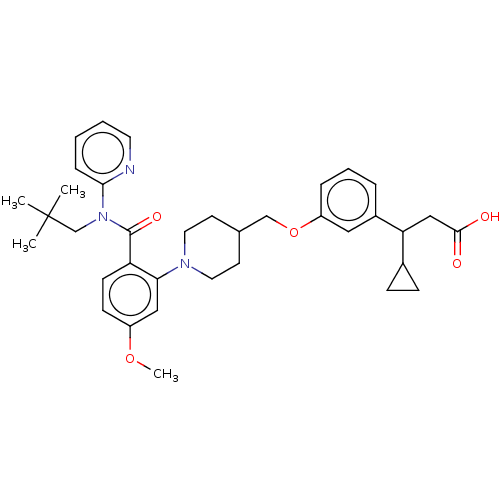 (CHEMBL4794401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561256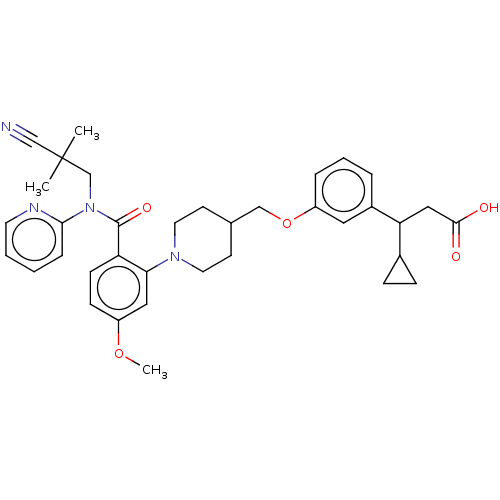 (CHEMBL4752289) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561257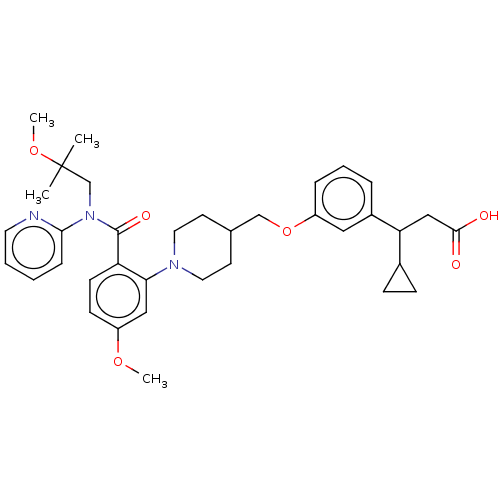 (CHEMBL4742099) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561258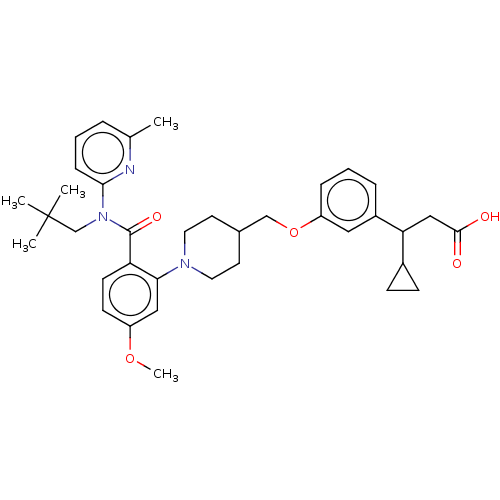 (CHEMBL4763105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561259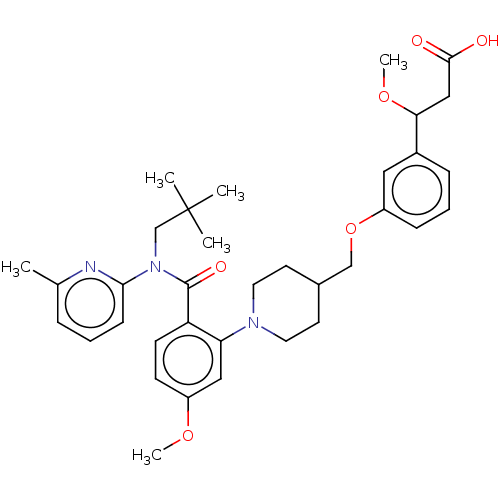 (CHEMBL4743254) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561260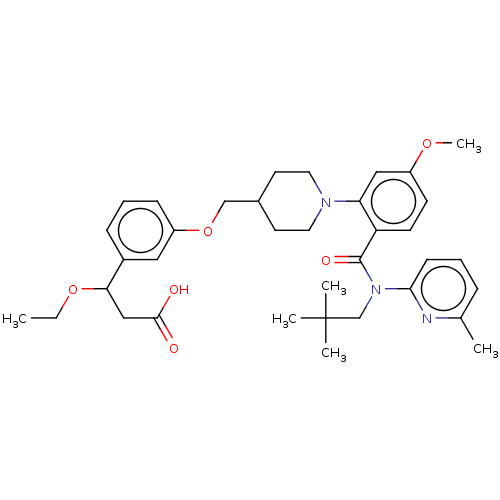 (CHEMBL4761155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561261 (CHEMBL4748658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561262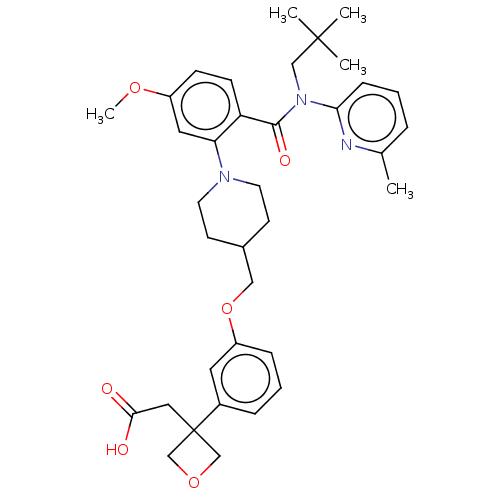 (CHEMBL4786019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561263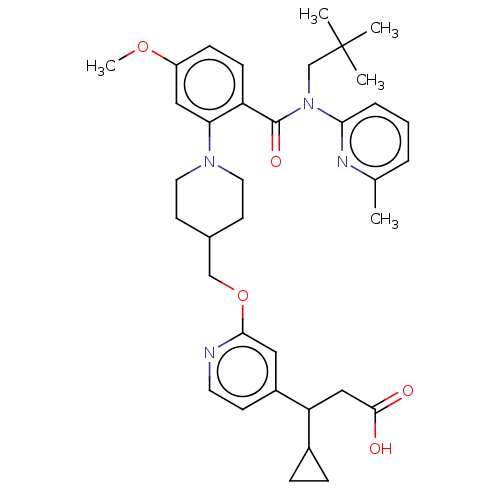 (CHEMBL4800194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561264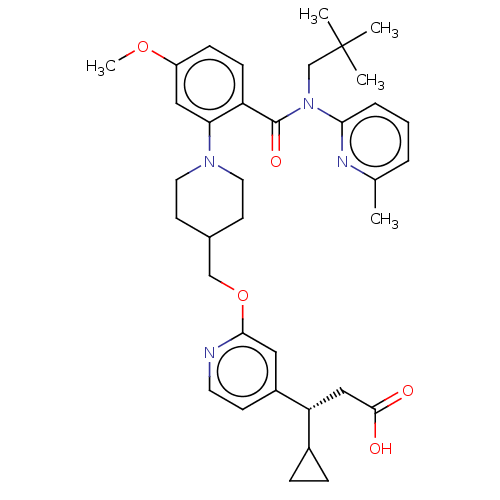 (CHEMBL4797024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50392861 (CHEMBL2152070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50386790 (CHEMBL1829174 | CHEMBL2047159 | TAK-875 | US119052...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561265 (CHEMBL4793427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50392861 (CHEMBL2152070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human GPR40 expressed in CHO cells by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50386790 (CHEMBL1829174 | CHEMBL2047159 | TAK-875 | US119052...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human GPR40 expressed in CHO cells by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50561241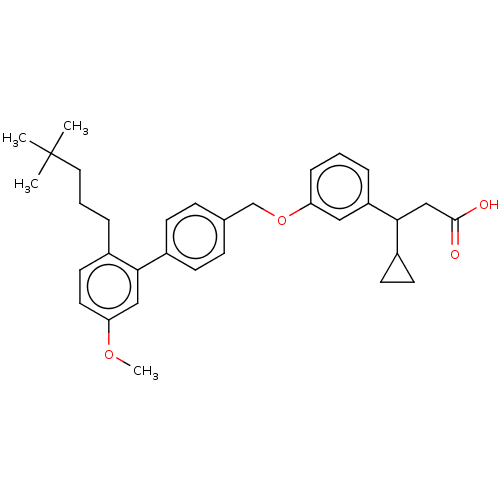 (CHEMBL4763356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR40 expressed in CHO cells incubated for 60 mins by FLIPR based Ca2+ mobilization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00843 BindingDB Entry DOI: 10.7270/Q2R2152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260529 (CHEMBL4077626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to biotinylated BCL6 (unknown origin) by SPR analysis | Bioorg Med Chem 25: 4876-4886 (2017) Article DOI: 10.1016/j.bmc.2017.07.037 BindingDB Entry DOI: 10.7270/Q2G44SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260534 (CHEMBL4066375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to recombinant RED-NHS labelled BCL6 BTB domain (1 to 129 residues) (unknown origin) after 10 mins by microscale thermophoresis meth... | Bioorg Med Chem 25: 4876-4886 (2017) Article DOI: 10.1016/j.bmc.2017.07.037 BindingDB Entry DOI: 10.7270/Q2G44SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |