 Found 312 hits with Last Name = 'zheng' and Initial = 'yg'
Found 312 hits with Last Name = 'zheng' and Initial = 'yg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50328743
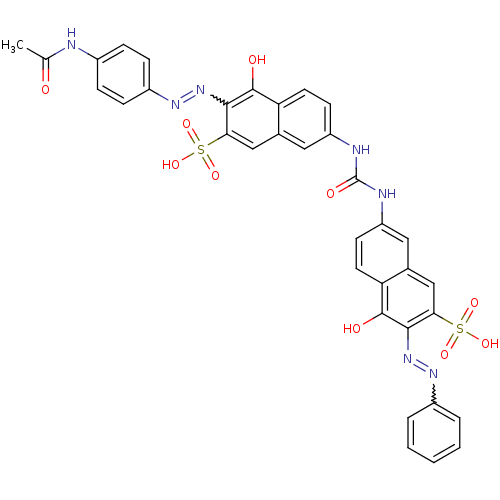
(3-((4-acetamidophenyl)diazenyl)-4-hydroxy-7-(3-(5-...)Show SMILES CC(=O)Nc1ccc(cc1)N=Nc1c(O)c2ccc(NC(=O)Nc3ccc4c(O)c(N=Nc5ccccc5)c(cc4c3)S(O)(=O)=O)cc2cc1S(O)(=O)=O |w:31.32,11.12| Show InChI InChI=1S/C35H27N7O10S2/c1-19(43)36-22-7-9-24(10-8-22)40-42-32-30(54(50,51)52)18-21-16-26(12-14-28(21)34(32)45)38-35(46)37-25-11-13-27-20(15-25)17-29(53(47,48)49)31(33(27)44)41-39-23-5-3-2-4-6-23/h2-18,44-45H,1H3,(H,36,43)(H2,37,38,46)(H,47,48,49)(H,50,51,52) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Binding affinity to His6x-tagged PRMT1 expressed in Escherichia coli BL21 (DE3) using fluorescein-labeled H4(1-20) peptide substrate by spectrofluoro... |
J Med Chem 53: 6028-39 (2010)
Article DOI: 10.1021/jm100416n
BindingDB Entry DOI: 10.7270/Q2251JDB |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397031

(CHEMBL2171190)Show SMILES [#8]-c1cc2ccccc2cc1-[#6](=O)-[#7]\[#7]=[#6]-1\[#6]=[#6]/[#6](/[#6]=[#6]-1)=[#7]\[#8]S(=O)(=O)c1ccccc1 |c:18,21| Show InChI InChI=1S/C23H17N3O5S/c27-22-15-17-7-5-4-6-16(17)14-21(22)23(28)25-24-18-10-12-19(13-11-18)26-31-32(29,30)20-8-2-1-3-9-20/h1-15,27H,(H,25,28)/b24-18-,26-19+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397030
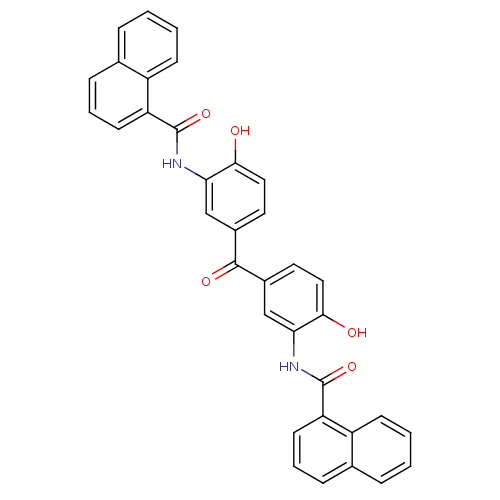
(CHEMBL2171189)Show SMILES Oc1ccc(cc1NC(=O)c1cccc2ccccc12)C(=O)c1ccc(O)c(NC(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C35H24N2O5/c38-31-17-15-23(19-29(31)36-34(41)27-13-5-9-21-7-1-3-11-25(21)27)33(40)24-16-18-32(39)30(20-24)37-35(42)28-14-6-10-22-8-2-4-12-26(22)28/h1-20,38-39H,(H,36,41)(H,37,42) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50210461
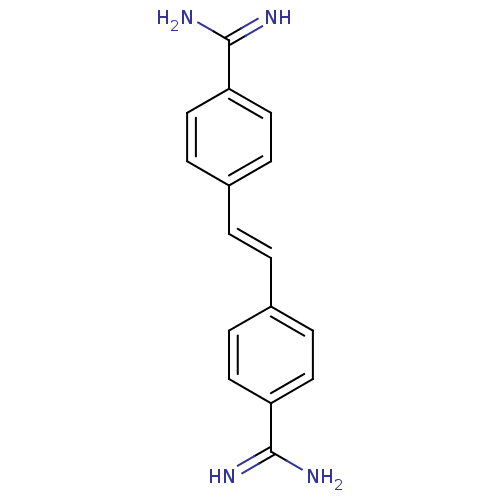
((stilbamidine)4-[2-[4-amino(imino)methylphenyl]-(E...)Show InChI InChI=1S/C16H16N4/c17-15(18)13-7-3-11(4-8-13)1-2-12-5-9-14(10-6-12)16(19)20/h1-10H,(H3,17,18)(H3,19,20)/b2-1+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of His6x-tagged PRMT1-mediated arginine methylation expressed in Escherichia coli BL21 (DE3) using H4(1-11) and [14C]-SAM by scintillation... |
J Med Chem 53: 6028-39 (2010)
Article DOI: 10.1021/jm100416n
BindingDB Entry DOI: 10.7270/Q2251JDB |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397030

(CHEMBL2171189)Show SMILES Oc1ccc(cc1NC(=O)c1cccc2ccccc12)C(=O)c1ccc(O)c(NC(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C35H24N2O5/c38-31-17-15-23(19-29(31)36-34(41)27-13-5-9-21-7-1-3-11-25(21)27)33(40)24-16-18-32(39)30(20-24)37-35(42)28-14-6-10-22-8-2-4-12-26(22)28/h1-20,38-39H,(H,36,41)(H,37,42) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50210461

((stilbamidine)4-[2-[4-amino(imino)methylphenyl]-(E...)Show InChI InChI=1S/C16H16N4/c17-15(18)13-7-3-11(4-8-13)1-2-12-5-9-14(10-6-12)16(19)20/h1-10H,(H3,17,18)(H3,19,20)/b2-1+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Binding affinity to His6x-tagged PRMT1 expressed in Escherichia coli BL21 (DE3) using fluorescein-labeled H4(1-11) peptide by spectrofluorometer anal... |
J Med Chem 53: 6028-39 (2010)
Article DOI: 10.1021/jm100416n
BindingDB Entry DOI: 10.7270/Q2251JDB |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397031

(CHEMBL2171190)Show SMILES [#8]-c1cc2ccccc2cc1-[#6](=O)-[#7]\[#7]=[#6]-1\[#6]=[#6]/[#6](/[#6]=[#6]-1)=[#7]\[#8]S(=O)(=O)c1ccccc1 |c:18,21| Show InChI InChI=1S/C23H17N3O5S/c27-22-15-17-7-5-4-6-16(17)14-21(22)23(28)25-24-18-10-12-19(13-11-18)26-31-32(29,30)20-8-2-1-3-9-20/h1-15,27H,(H,25,28)/b24-18-,26-19+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50243388

(AT-9283 | US10981896, Compound AT9283)Show SMILES O=C(NC1CC1)Nc1c[nH]nc1-c1nc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C19H23N7O2/c27-19(21-13-2-3-13)24-16-10-20-25-17(16)18-22-14-4-1-12(9-15(14)23-18)11-26-5-7-28-8-6-26/h1,4,9-10,13H,2-3,5-8,11H2,(H,20,25)(H,22,23)(H2,21,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50243388

(AT-9283 | US10981896, Compound AT9283)Show SMILES O=C(NC1CC1)Nc1c[nH]nc1-c1nc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C19H23N7O2/c27-19(21-13-2-3-13)24-16-10-20-25-17(16)18-22-14-4-1-12(9-15(14)23-18)11-26-5-7-28-8-6-26/h1,4,9-10,13H,2-3,5-8,11H2,(H,20,25)(H,22,23)(H2,21,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602353
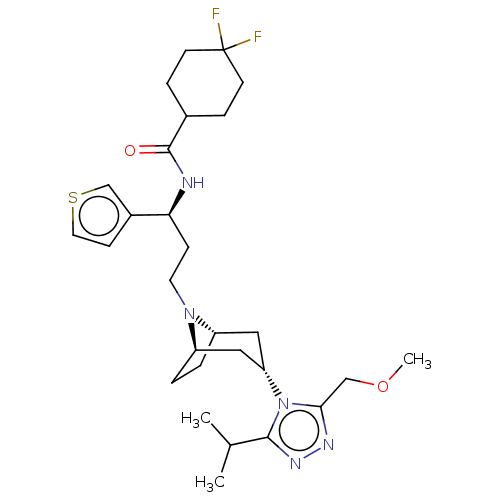
(CHEMBL5171331)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(COC)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccsc1 |r,TLB:9:7:3.2:20| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237553
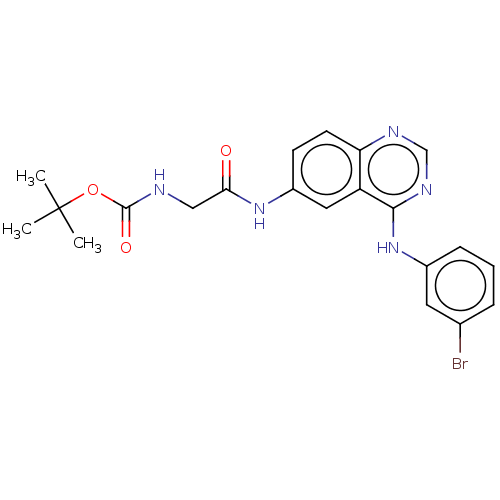
(CHEMBL4071058)Show SMILES CC(C)(C)OC(=O)NCC(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 Show InChI InChI=1S/C21H22BrN5O3/c1-21(2,3)30-20(29)23-11-18(28)26-15-7-8-17-16(10-15)19(25-12-24-17)27-14-6-4-5-13(22)9-14/h4-10,12H,11H2,1-3H3,(H,23,29)(H,26,28)(H,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602350
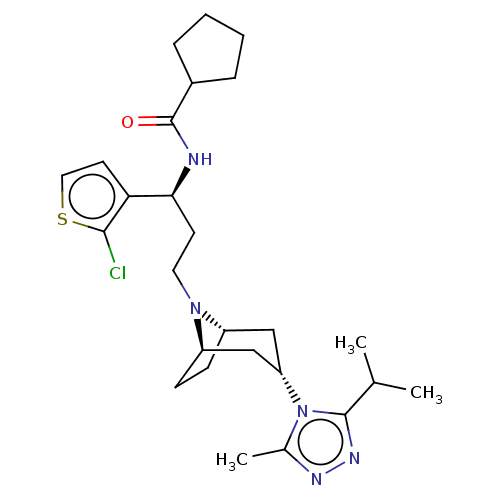
(CHEMBL5180628)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCCC1)c1ccsc1Cl |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602351
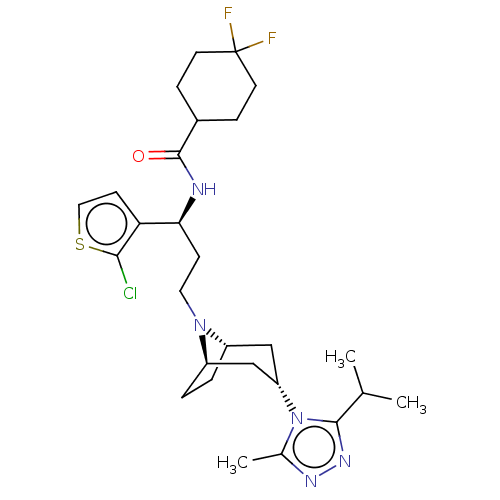
(CHEMBL5177519)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccsc1Cl |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602343
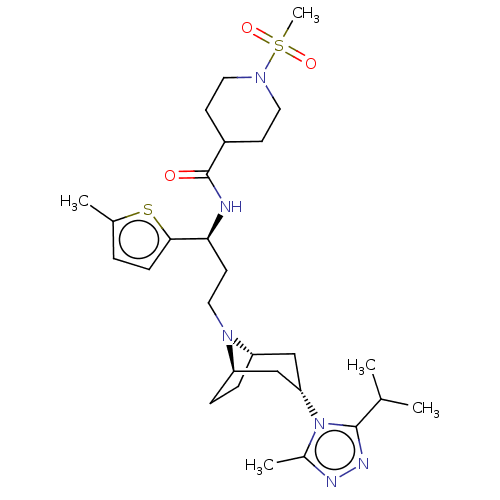
(CHEMBL5205216)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCN(CC1)S(C)(=O)=O)c1ccc(C)s1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50556682
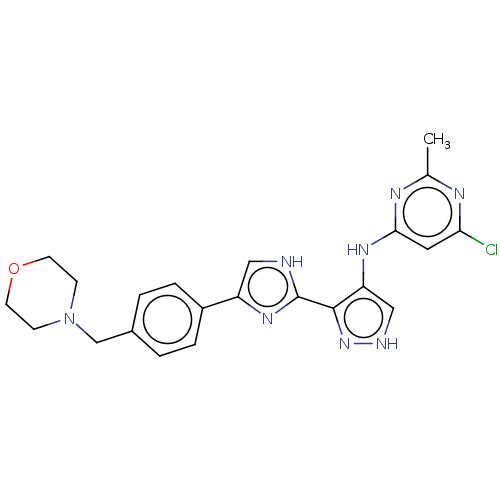
(CHEMBL4794655)Show SMILES Cc1nc(Cl)cc(Nc2c[nH]nc2-c2nc(c[nH]2)-c2ccc(CN3CCOCC3)cc2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50334986

(4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...)Show SMILES CC(C)c1nnc(C)n1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602354
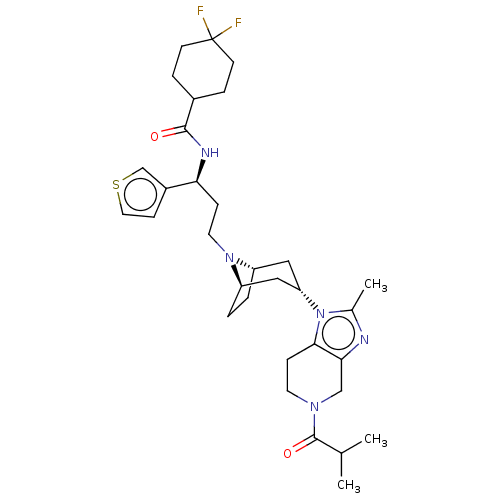
(CHEMBL5209067)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nc3CN(CCc13)C(=O)C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccsc1 |r,TLB:9:7:3.2:24| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602349
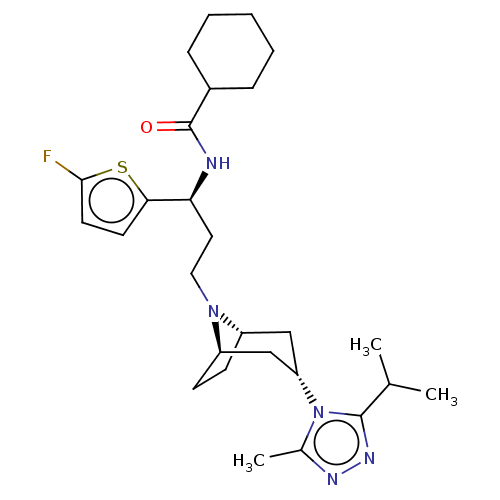
(CHEMBL5200961)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCCCC1)c1ccc(F)s1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602340
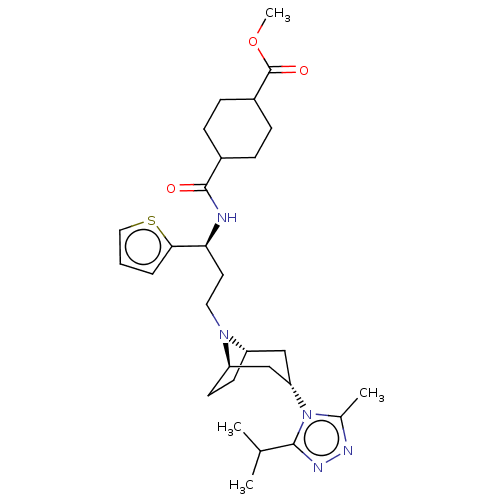
(CHEMBL5185201)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(CC1)C(=O)OC)c1cccs1 |r,wU:21.24,1.0,wD:7.9,4.4,TLB:9:7:3.2:18,(1.2,-2.21,;.8,-3.7,;-.03,-5,;.19,-6.5,;1.05,-5.24,;1.82,-6.57,;2.51,-4.87,;4.33,-5.03,;2.25,-3.4,;5.82,-5.43,;6.91,-4.34,;6.51,-2.86,;8.3,-5.08,;8.06,-6.56,;6.53,-6.79,;5.76,-8.12,;6.53,-9.46,;4.22,-8.12,;-.65,-2.73,;-1.74,-1.64,;-3.22,-2.04,;-4.31,-.95,;-3.91,.54,;-5,1.63,;-6.49,1.23,;-4.6,3.11,;-5.69,4.2,;-5.29,5.69,;-3.81,6.08,;-2.72,5,;-3.11,3.51,;-3.41,7.57,;-4.5,8.66,;-1.92,7.97,;-1.52,9.46,;-5.8,-1.35,;-7.09,-.53,;-8.3,-1.49,;-7.77,-2.9,;-6.2,-2.84,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50556681
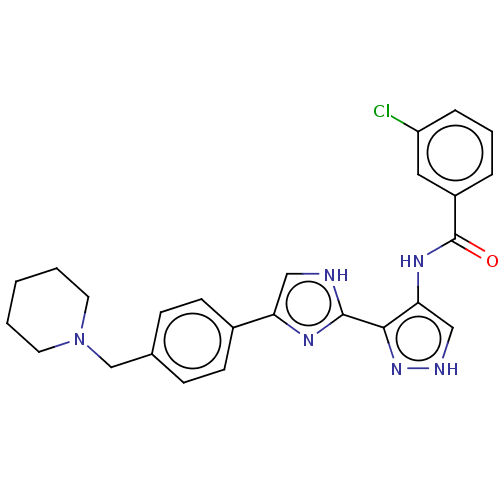
(CHEMBL4795804)Show SMILES Clc1cccc(c1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCCCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50556677
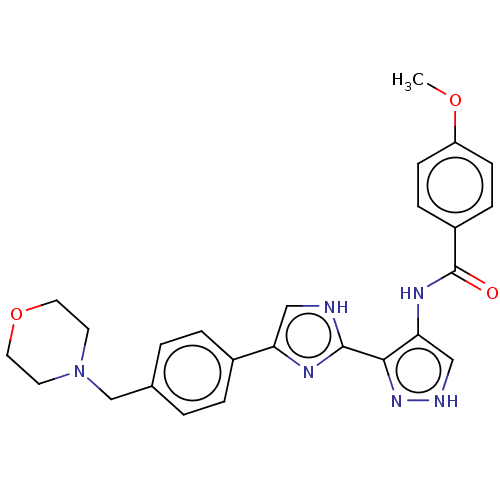
(CHEMBL4782433)Show SMILES COc1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602336
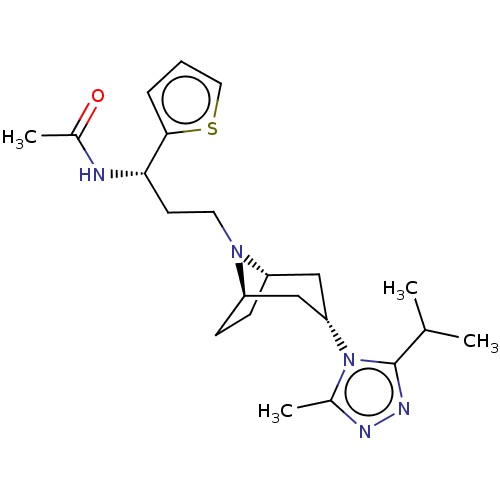
(CHEMBL5176665)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(C)=O)c1cccs1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602355
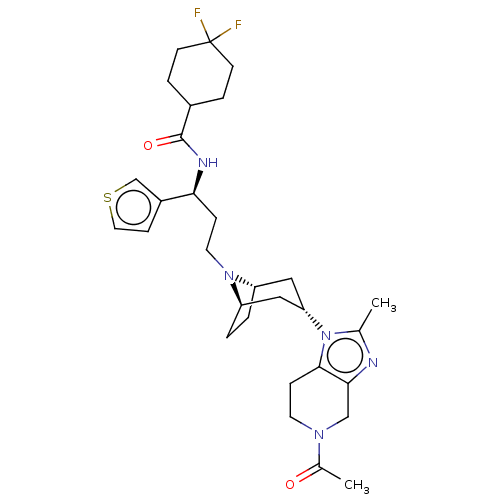
(CHEMBL5175220)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nc3CN(CCc13)C(C)=O)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccsc1 |r,TLB:9:7:3.2:22| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50556681

(CHEMBL4795804)Show SMILES Clc1cccc(c1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCCCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237554
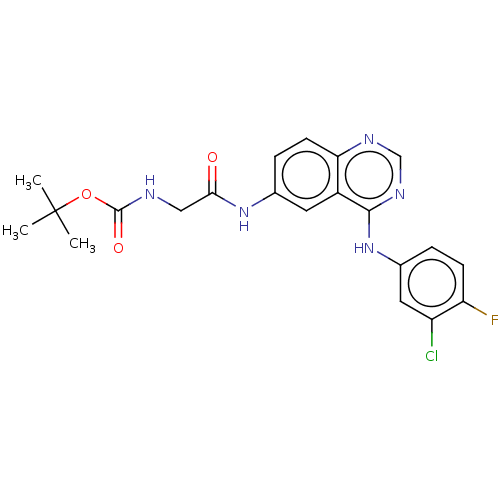
(CHEMBL4098587)Show SMILES CC(C)(C)OC(=O)NCC(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C21H21ClFN5O3/c1-21(2,3)31-20(30)24-10-18(29)27-12-5-7-17-14(8-12)19(26-11-25-17)28-13-4-6-16(23)15(22)9-13/h4-9,11H,10H2,1-3H3,(H,24,30)(H,27,29)(H,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602345
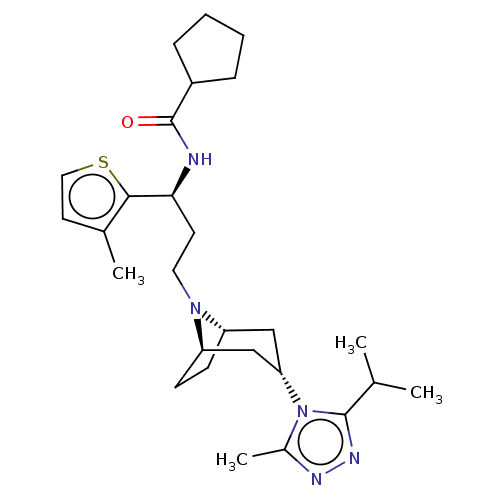
(CHEMBL5178121)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCCC1)c1sccc1C |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM314060
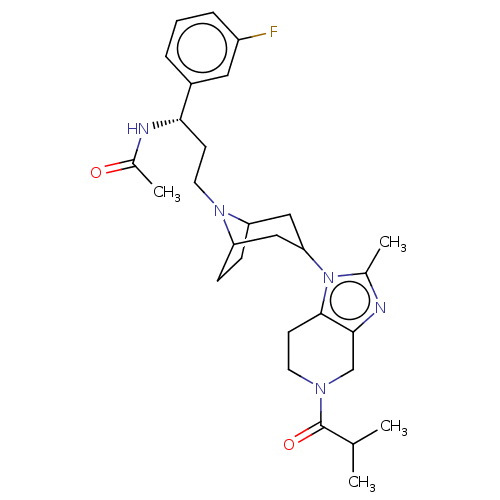
(US10167299, PF-232798The)Show SMILES CC(C)C(=O)N1CCc2c(C1)nc(C)n2C1CC2CCC(C1)N2CC[C@H](NC(C)=O)c1cccc(F)c1 |r,TLB:23:22:16.15.21:18.19,14:15:22:18.19| Show InChI InChI=1S/C29H40FN5O2/c1-18(2)29(37)33-12-11-28-27(17-33)31-19(3)35(28)25-15-23-8-9-24(16-25)34(23)13-10-26(32-20(4)36)21-6-5-7-22(30)14-21/h5-7,14,18,23-26H,8-13,15-17H2,1-4H3,(H,32,36)/t23?,24?,25?,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602344
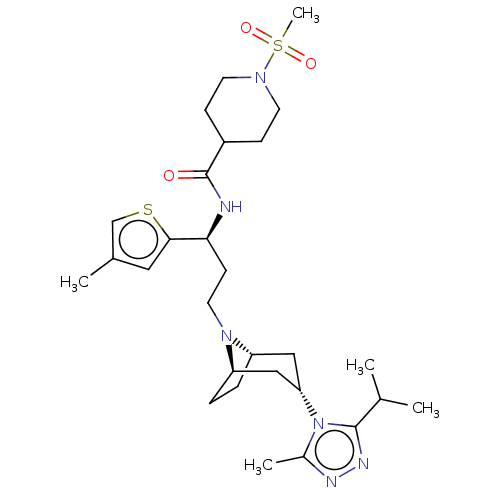
(CHEMBL5204027)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCN(CC1)S(C)(=O)=O)c1cc(C)cs1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50556682

(CHEMBL4794655)Show SMILES Cc1nc(Cl)cc(Nc2c[nH]nc2-c2nc(c[nH]2)-c2ccc(CN3CCOCC3)cc2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50556677

(CHEMBL4782433)Show SMILES COc1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50243388

(AT-9283 | US10981896, Compound AT9283)Show SMILES O=C(NC1CC1)Nc1c[nH]nc1-c1nc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C19H23N7O2/c27-19(21-13-2-3-13)24-16-10-20-25-17(16)18-22-14-4-1-12(9-15(14)23-18)11-26-5-7-28-8-6-26/h1,4,9-10,13H,2-3,5-8,11H2,(H,20,25)(H,22,23)(H2,21,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora A (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602339
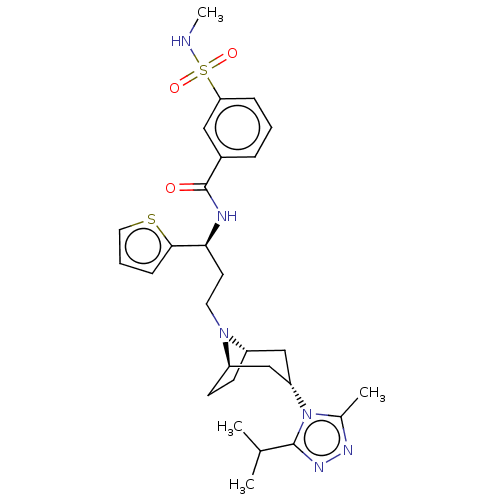
(CHEMBL5197247)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)c1cccc(c1)S(=O)(=O)NC)c1cccs1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602337
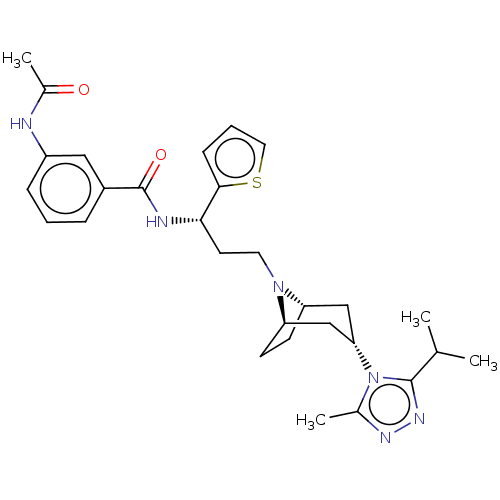
(CHEMBL5178495)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)c1cccc(NC(C)=O)c1)c1cccs1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602342
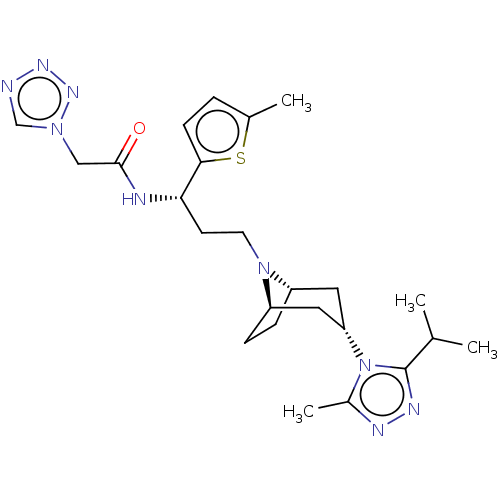
(CHEMBL5208613)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)Cn1cnnn1)c1ccc(C)s1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237602
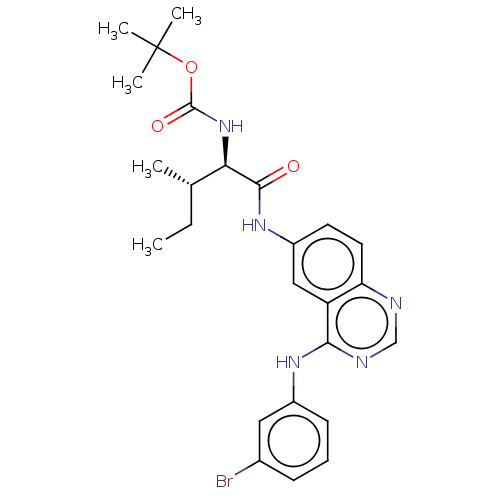
(CHEMBL4061283)Show SMILES CC[C@H](C)[C@@H](NC(=O)OC(C)(C)C)C(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 |r| Show InChI InChI=1S/C25H30BrN5O3/c1-6-15(2)21(31-24(33)34-25(3,4)5)23(32)30-18-10-11-20-19(13-18)22(28-14-27-20)29-17-9-7-8-16(26)12-17/h7-15,21H,6H2,1-5H3,(H,30,32)(H,31,33)(H,27,28,29)/t15-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50556680

(CHEMBL4758261)Show SMILES Clc1cccc(c1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50243388

(AT-9283 | US10981896, Compound AT9283)Show SMILES O=C(NC1CC1)Nc1c[nH]nc1-c1nc2cc(CN3CCOCC3)ccc2[nH]1 Show InChI InChI=1S/C19H23N7O2/c27-19(21-13-2-3-13)24-16-10-20-25-17(16)18-22-14-4-1-12(9-15(14)23-18)11-26-5-7-28-8-6-26/h1,4,9-10,13H,2-3,5-8,11H2,(H,20,25)(H,22,23)(H2,21,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora B (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50556679
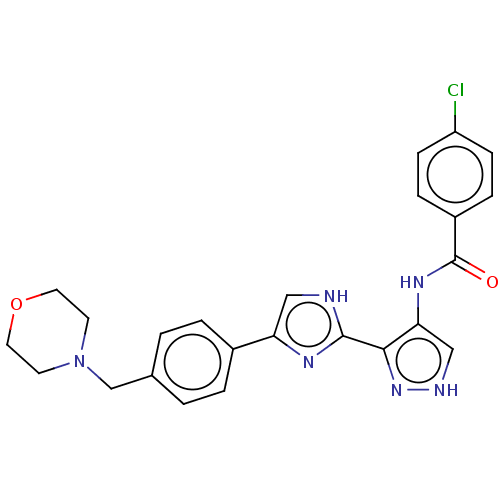
(CHEMBL4763483)Show SMILES Clc1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50464151
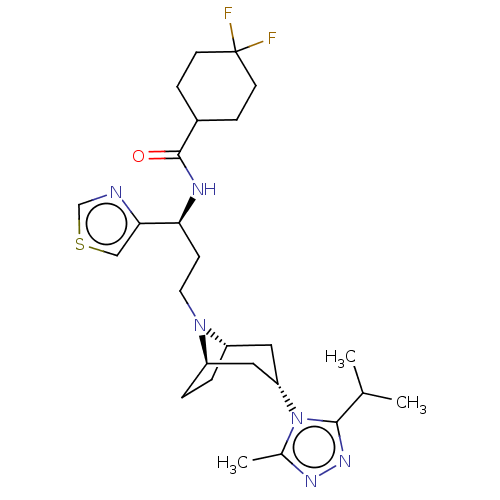
(CHEMBL4240742)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1cscn1 |r,TLB:19:18:7.6.8:3.2,THB:9:7:18:3.2| Show InChI InChI=1S/C26H38F2N6OS/c1-16(2)24-32-31-17(3)34(24)21-12-19-4-5-20(13-21)33(19)11-8-22(23-14-36-15-29-23)30-25(35)18-6-9-26(27,28)10-7-18/h14-16,18-22H,4-13H2,1-3H3,(H,30,35)/t19-,20+,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50602341
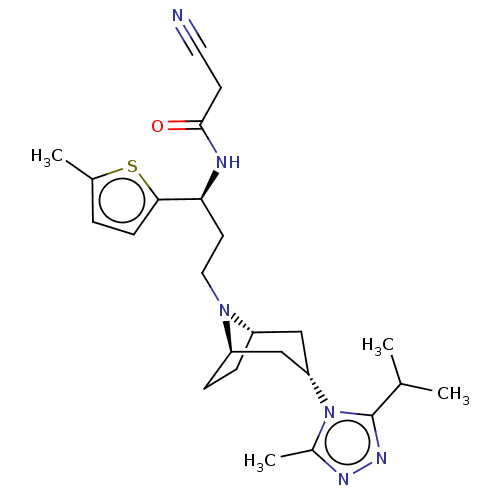
(CHEMBL5205626)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)n1c(C)nnc1C(C)C)N2CC[C@H](NC(=O)CC#N)c1ccc(C)s1 |r,TLB:9:7:3.2:18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01383
BindingDB Entry DOI: 10.7270/Q2CZ3C7H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50556679

(CHEMBL4763483)Show SMILES Clc1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237556
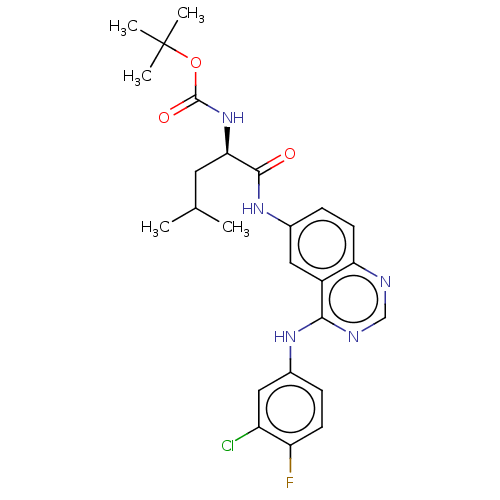
(CHEMBL4087241)Show SMILES CC(C)C[C@@H](NC(=O)OC(C)(C)C)C(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 |r| Show InChI InChI=1S/C25H29ClFN5O3/c1-14(2)10-21(32-24(34)35-25(3,4)5)23(33)31-15-7-9-20-17(11-15)22(29-13-28-20)30-16-6-8-19(27)18(26)12-16/h6-9,11-14,21H,10H2,1-5H3,(H,31,33)(H,32,34)(H,28,29,30)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237550
(CHEMBL4091010)Show SMILES CC(C)C[C@@H](NC(=O)OC(C)(C)C)C(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 |r| Show InChI InChI=1S/C25H30BrN5O3/c1-15(2)11-21(31-24(33)34-25(3,4)5)23(32)30-18-9-10-20-19(13-18)22(28-14-27-20)29-17-8-6-7-16(26)12-17/h6-10,12-15,21H,11H2,1-5H3,(H,30,32)(H,31,33)(H,27,28,29)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50556682

(CHEMBL4794655)Show SMILES Cc1nc(Cl)cc(Nc2c[nH]nc2-c2nc(c[nH]2)-c2ccc(CN3CCOCC3)cc2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora A (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) measured after 40 mins by Kinase Glo luminescence assay |
Eur J Med Chem 130: 393-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.061
BindingDB Entry DOI: 10.7270/Q21Z46PJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50556680

(CHEMBL4758261)Show SMILES Clc1cccc(c1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50556677

(CHEMBL4782433)Show SMILES COc1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora A (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Georgia
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SAM from recombinant His6-tagged PRMT1 (unknown origin) expressed in Escherichia coli BL21(DE3) incubated for 5 mins prior to H4... |
J Med Chem 58: 1228-43 (2015)
Article DOI: 10.1021/jm501452j
BindingDB Entry DOI: 10.7270/Q29S1SQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50556678
(CHEMBL4744513)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)Nc1c[nH]nc1-c1nc(c[nH]1)-c1ccc(CN2CCOCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 466 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora A (unknown origin) incubated for 40 mins in presence of ATP by Kinase-glo luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112934
BindingDB Entry DOI: 10.7270/Q2H70KG0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data