 Found 74 hits with Last Name = 'chen' and Initial = 'yq'
Found 74 hits with Last Name = 'chen' and Initial = 'yq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449386
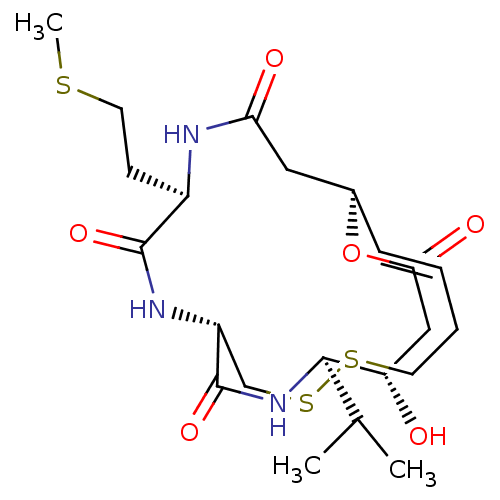
(CHEMBL3126833)Show SMILES CSCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)C(C)C |r,t:26| Show InChI InChI=1S/C22H35N3O6S3/c1-13(2)20-17(26)11-19(28)31-14-6-4-5-8-33-34-12-16(22(30)25-20)24-21(29)15(7-9-32-3)23-18(27)10-14/h4,6,13-17,20,26H,5,7-12H2,1-3H3,(H,23,27)(H,24,29)(H,25,30)/b6-4+/t14-,15-,16-,17+,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354088
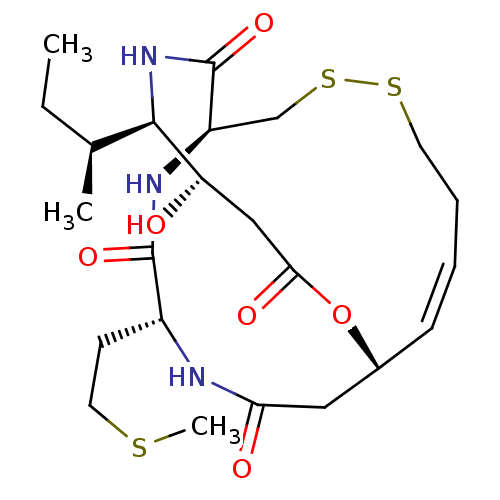
(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19151
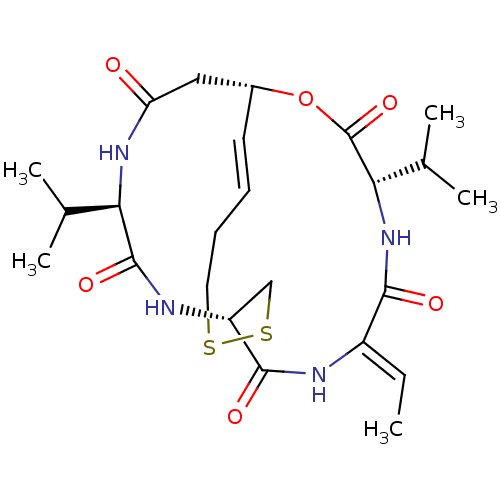
((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50496770
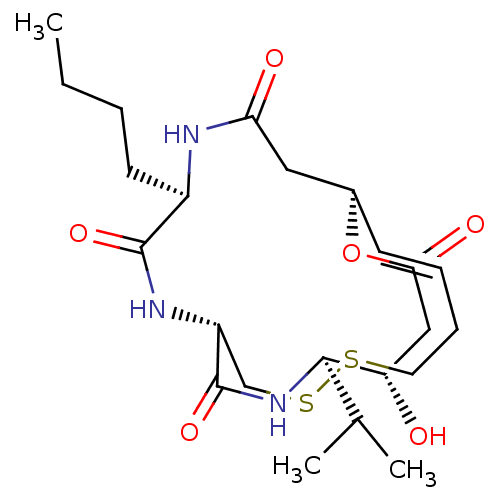
(CHEMBL3218562)Show SMILES [H][C@]12CC(=O)N[C@H](CCCC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](C(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C23H37N3O6S2/c1-4-5-9-16-22(30)25-17-13-34-33-10-7-6-8-15(11-19(28)24-16)32-20(29)12-18(27)21(14(2)3)26-23(17)31/h6,8,14-18,21,27H,4-5,7,9-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b8-6+/t15-,16-,17-,18+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50496768
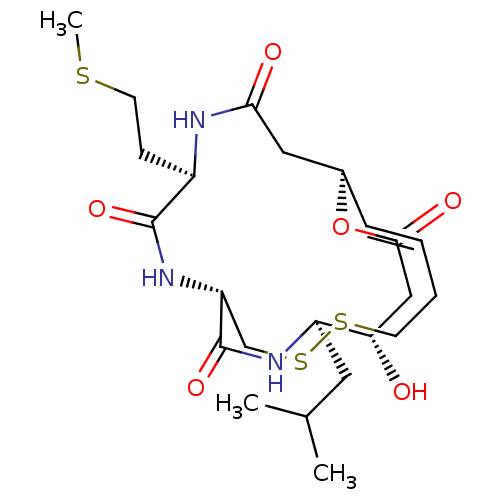
(CHEMBL3218563)Show SMILES [H][C@]12CC(=O)N[C@H](CCSC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](CC(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C23H37N3O6S3/c1-14(2)10-17-19(27)12-21(29)32-15-6-4-5-8-34-35-13-18(23(31)25-17)26-22(30)16(7-9-33-3)24-20(28)11-15/h4,6,14-19,27H,5,7-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,30)/b6-4+/t15-,16-,17-,18-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354084
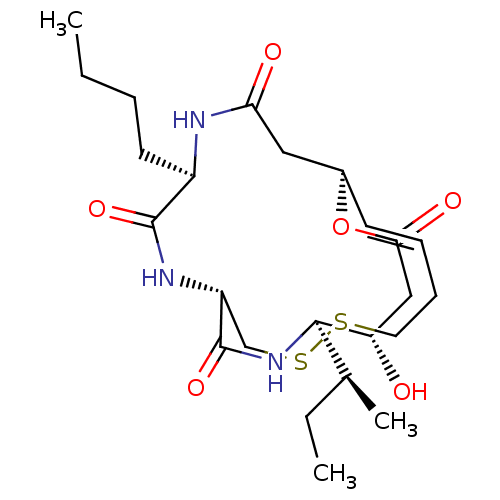
(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50496769
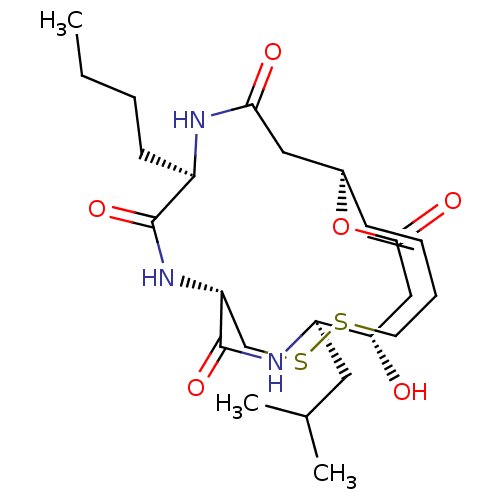
(CHEMBL3218564)Show SMILES [H][C@]12CC(=O)N[C@H](CCCC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](CC(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C24H39N3O6S2/c1-4-5-9-17-23(31)27-19-14-35-34-10-7-6-8-16(12-21(29)25-17)33-22(30)13-20(28)18(11-15(2)3)26-24(19)32/h6,8,15-20,28H,4-5,7,9-14H2,1-3H3,(H,25,29)(H,26,32)(H,27,31)/b8-6+/t16-,17-,18-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354089
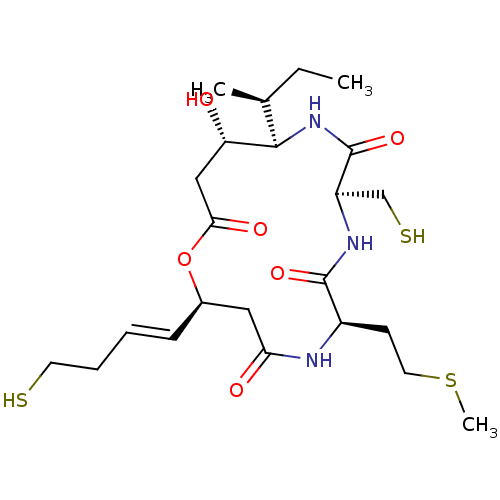
(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354087
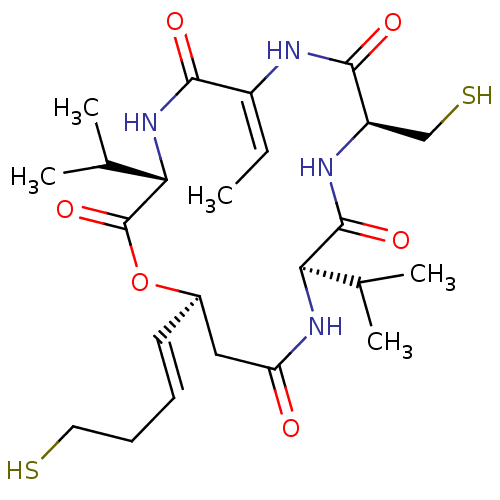
(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354085
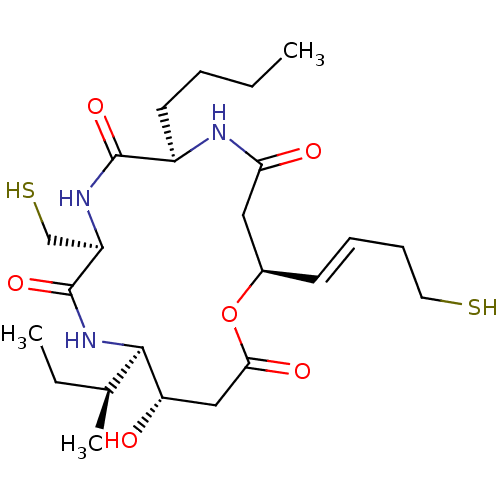
(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50354087

(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 1... |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50354088

(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354087

(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354089

(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50354087

(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354085

(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19151

((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50449386

(CHEMBL3126833)Show SMILES CSCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)C(C)C |r,t:26| Show InChI InChI=1S/C22H35N3O6S3/c1-13(2)20-17(26)11-19(28)31-14-6-4-5-8-33-34-12-16(22(30)25-20)24-21(29)15(7-9-32-3)23-18(27)10-14/h4,6,13-17,20,26H,5,7-12H2,1-3H3,(H,23,27)(H,24,29)(H,25,30)/b6-4+/t14-,15-,16-,17+,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354088

(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50496770

(CHEMBL3218562)Show SMILES [H][C@]12CC(=O)N[C@H](CCCC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](C(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C23H37N3O6S2/c1-4-5-9-16-22(30)25-17-13-34-33-10-7-6-8-15(11-19(28)24-16)32-20(29)12-18(27)21(14(2)3)26-23(17)31/h6,8,14-18,21,27H,4-5,7,9-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b8-6+/t15-,16-,17-,18+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50354085

(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50354089

(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50496768

(CHEMBL3218563)Show SMILES [H][C@]12CC(=O)N[C@H](CCSC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](CC(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C23H37N3O6S3/c1-14(2)10-17-19(27)12-21(29)32-15-6-4-5-8-34-35-13-18(23(31)25-17)26-22(30)16(7-9-33-3)24-20(28)11-15/h4,6,14-19,27H,5,7-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,30)/b6-4+/t15-,16-,17-,18-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50496769

(CHEMBL3218564)Show SMILES [H][C@]12CC(=O)N[C@H](CCCC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](CC(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C24H39N3O6S2/c1-4-5-9-17-23(31)27-19-14-35-34-10-7-6-8-16(12-21(29)25-17)33-22(30)13-20(28)18(11-15(2)3)26-24(19)32/h6,8,15-20,28H,4-5,7,9-14H2,1-3H3,(H,25,29)(H,26,32)(H,27,31)/b8-6+/t16-,17-,18-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50354087

(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19151

((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354088

(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50354089

(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(epsilon-acetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50354087

(CHEMBL1836143)Show SMILES C\C=C1\NC(=O)[C@@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6+/t15-,17-,19-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50354089

(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50354085

(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50354085

(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC7 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50354084

(CHEMBL1836145)Show SMILES CCCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)[C@@H](C)CC |r,t:26| Show InChI InChI=1S/C24H39N3O6S2/c1-4-6-10-17-23(31)26-18-14-35-34-11-8-7-9-16(12-20(29)25-17)33-21(30)13-19(28)22(15(3)5-2)27-24(18)32/h7,9,15-19,22,28H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50354085

(CHEMBL1836042)Show SMILES CCCC[C@H]1NC(=O)C[C@H](OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CS)NC1=O)[C@@H](C)CC)\C=C\CCS |r| Show InChI InChI=1S/C24H41N3O6S2/c1-4-6-10-17-23(31)26-18(14-35)24(32)27-22(15(3)5-2)19(28)13-21(30)33-16(9-7-8-11-34)12-20(29)25-17/h7,9,15-19,22,28,34-35H,4-6,8,10-14H2,1-3H3,(H,25,29)(H,26,31)(H,27,32)/b9-7+/t15-,16+,17+,18+,19-,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50354088

(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50449386

(CHEMBL3126833)Show SMILES CSCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)C(C)C |r,t:26| Show InChI InChI=1S/C22H35N3O6S3/c1-13(2)20-17(26)11-19(28)31-14-6-4-5-8-33-34-12-16(22(30)25-20)24-21(29)15(7-9-32-3)23-18(27)10-14/h4,6,13-17,20,26H,5,7-12H2,1-3H3,(H,23,27)(H,24,29)(H,25,30)/b6-4+/t14-,15-,16-,17+,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50354089

(CHEMBL1836144)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@@H](CS)NC(=O)[C@@H](CCSC)NC(=O)C[C@H](OC(=O)C[C@@H]1O)\C=C\CCS |r| Show InChI InChI=1S/C23H39N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15(7-5-6-9-33)11-19(28)24-16(8-10-35-3)22(30)25-17(13-34)23(31)26-21/h5,7,14-18,21,27,33-34H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC4 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50496770

(CHEMBL3218562)Show SMILES [H][C@]12CC(=O)N[C@H](CCCC)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N[C@H](C(C)C)[C@@H](O)CC(=O)O2 |r,t:21| Show InChI InChI=1S/C23H37N3O6S2/c1-4-5-9-16-22(30)25-17-13-34-33-10-7-6-8-15(11-19(28)24-16)32-20(29)12-18(27)21(14(2)3)26-23(17)31/h6,8,14-18,21,27H,4-5,7,9-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b8-6+/t15-,16-,17-,18+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50354088

(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC9 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay |
J Nat Prod 74: 2031-8 (2011)
Article DOI: 10.1021/np200324x
BindingDB Entry DOI: 10.7270/Q2MW2HJ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data