 Found 2401 hits with Last Name = 'chao' and Initial = 'ys'
Found 2401 hits with Last Name = 'chao' and Initial = 'ys' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326392

((2S)-1-({(2S,4S)-4-[2-(1,3-Dihydro-2H-isoindol-2-y...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)[C@@H]1C[C@@H](CC(=O)N2Cc3ccccc3C2)C(=O)N1 |r| Show InChI InChI=1S/C20H20F2N4O3/c21-20(22)7-15(8-23)26(11-20)19(29)16-5-14(18(28)24-16)6-17(27)25-9-12-3-1-2-4-13(12)10-25/h1-4,14-16H,5-7,9-11H2,(H,24,28)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326373
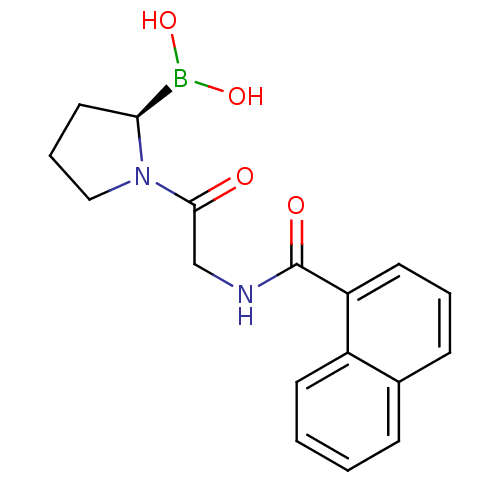
((R)-1-(2-(1-naphthamido)acetyl)pyrrolidin-2-ylboro...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C17H19BN2O4/c21-16(20-10-4-9-15(20)18(23)24)11-19-17(22)14-8-3-6-12-5-1-2-7-13(12)14/h1-3,5-8,15,23-24H,4,9-11H2,(H,19,22)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
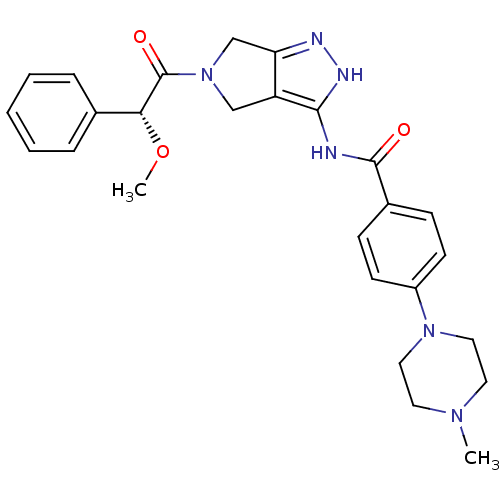
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM12985

(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM12985

(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055967
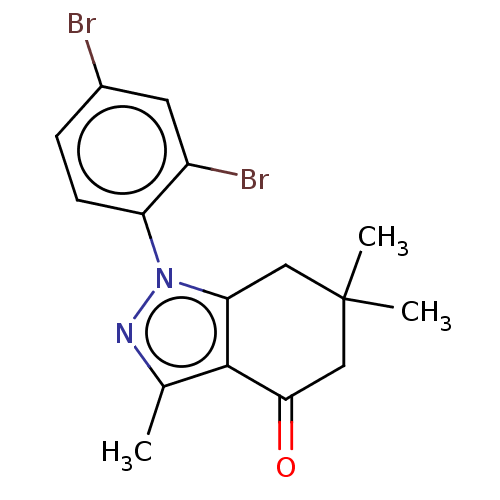
(CHEMBL3325714)Show InChI InChI=1S/C16H16Br2N2O/c1-9-15-13(7-16(2,3)8-14(15)21)20(19-9)12-5-4-10(17)6-11(12)18/h4-6H,7-8H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055966
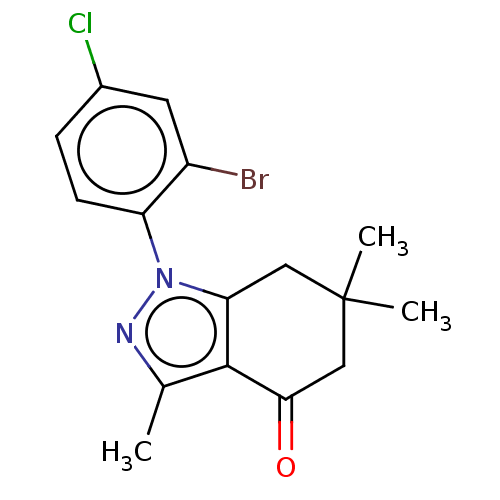
(CHEMBL3325715)Show InChI InChI=1S/C16H16BrClN2O/c1-9-15-13(7-16(2,3)8-14(15)21)20(19-9)12-5-4-10(18)6-11(12)17/h4-6H,7-8H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055965
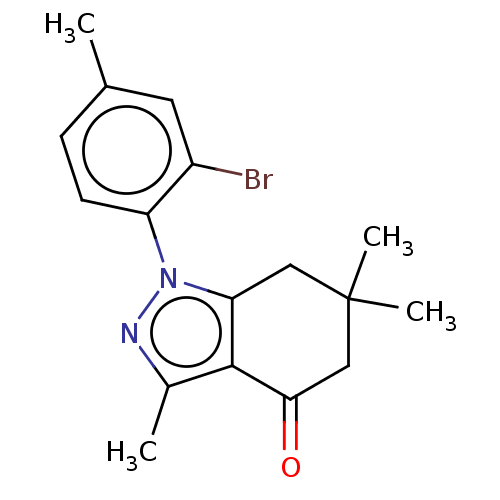
(CHEMBL3325716)Show InChI InChI=1S/C17H19BrN2O/c1-10-5-6-13(12(18)7-10)20-14-8-17(3,4)9-15(21)16(14)11(2)19-20/h5-7H,8-9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055969
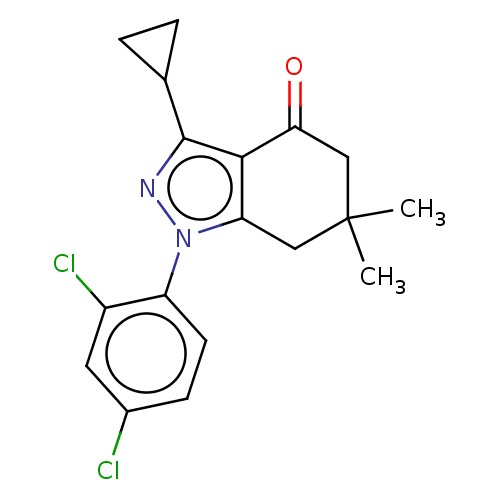
(CHEMBL3325719)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(Cl)cc2Cl)C2CC2)C(=O)C1 Show InChI InChI=1S/C18H18Cl2N2O/c1-18(2)8-14-16(15(23)9-18)17(10-3-4-10)21-22(14)13-6-5-11(19)7-12(13)20/h5-7,10H,3-4,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055973
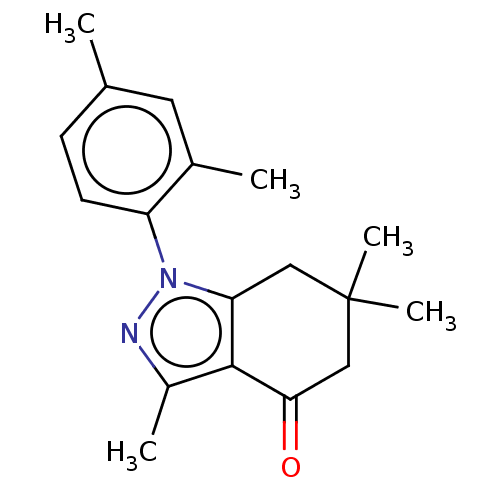
(CHEMBL3325707)Show InChI InChI=1S/C18H22N2O/c1-11-6-7-14(12(2)8-11)20-15-9-18(4,5)10-16(21)17(15)13(3)19-20/h6-8H,9-10H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM21973
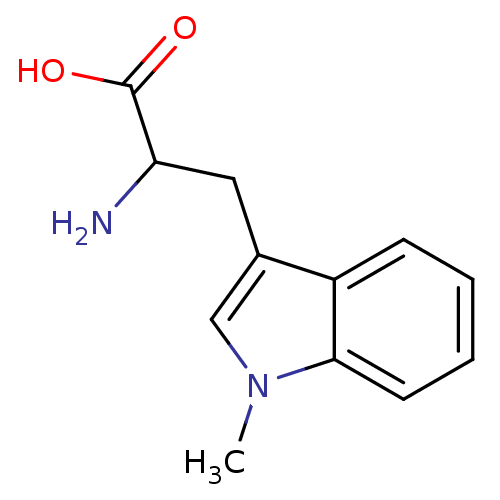
(1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO using L-tryptophan as substrate |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055972
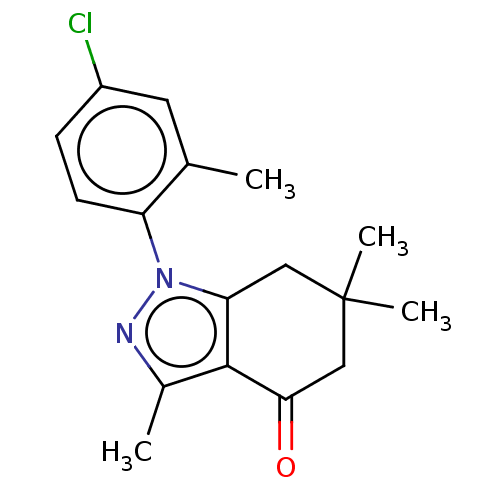
(CHEMBL3325710)Show InChI InChI=1S/C17H19ClN2O/c1-10-7-12(18)5-6-13(10)20-14-8-17(3,4)9-15(21)16(14)11(2)19-20/h5-7H,8-9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50055964
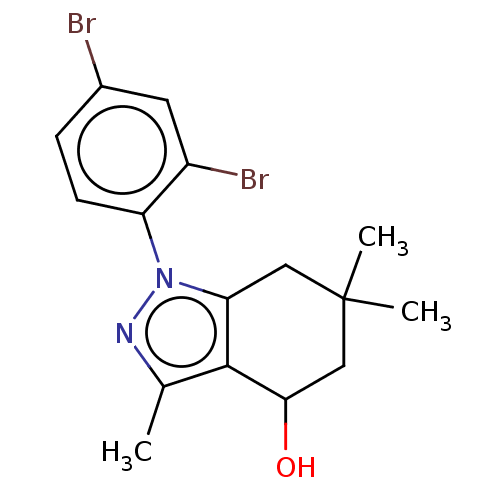
(CHEMBL3325841)Show InChI InChI=1S/C16H18Br2N2O/c1-9-15-13(7-16(2,3)8-14(15)21)20(19-9)12-5-4-10(17)6-11(12)18/h4-6,14,21H,7-8H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167698
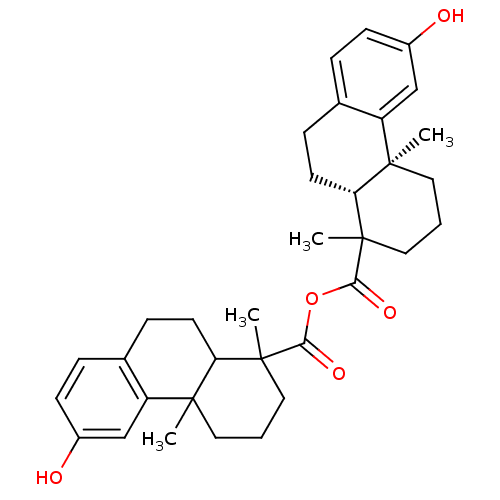
(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389234
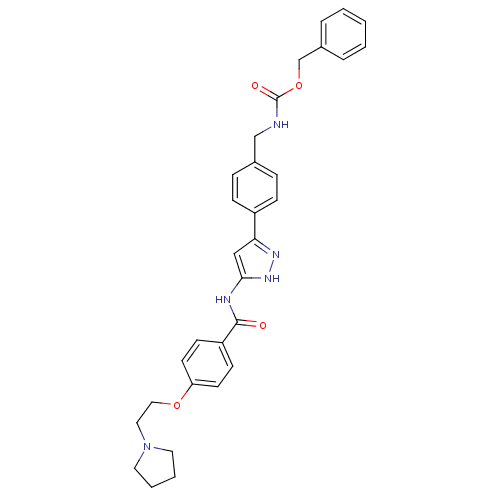
(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253758
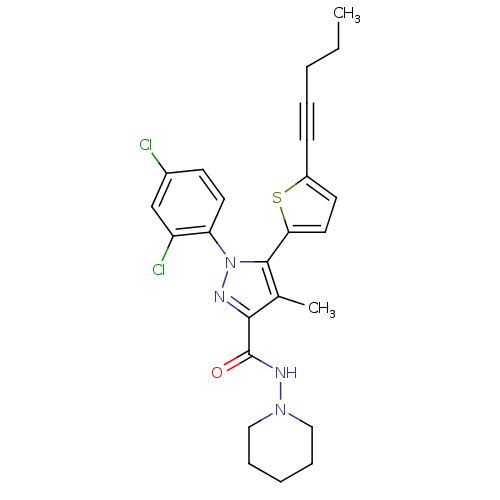
(1-(2,4-Dichlorophenyl)-4-methyl-5-(5-(pent-1-ynyl)...)Show SMILES CCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H26Cl2N4OS/c1-3-4-6-9-19-11-13-22(33-19)24-17(2)23(25(32)29-30-14-7-5-8-15-30)28-31(24)21-12-10-18(26)16-20(21)27/h10-13,16H,3-5,7-8,14-15H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253843
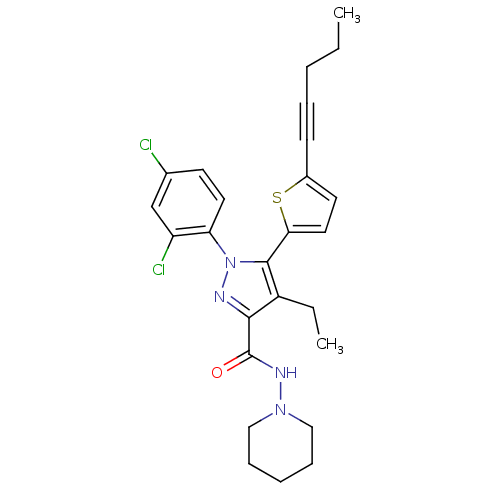
(1-(2,4-Dichlorophenyl)-4-ethyl-5-(5-(pent-1-ynyl)t...)Show SMILES CCCC#Cc1ccc(s1)-c1c(CC)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H28Cl2N4OS/c1-3-5-7-10-19-12-14-23(34-19)25-20(4-2)24(26(33)30-31-15-8-6-9-16-31)29-32(25)22-13-11-18(27)17-21(22)28/h11-14,17H,3-6,8-9,15-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253807
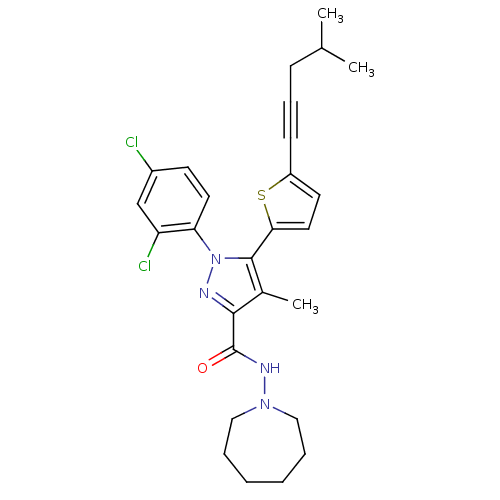
(CHEMBL462686 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...)Show SMILES CC(C)CC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C27H30Cl2N4OS/c1-18(2)9-8-10-21-12-14-24(35-21)26-19(3)25(27(34)31-32-15-6-4-5-7-16-32)30-33(26)23-13-11-20(28)17-22(23)29/h11-14,17-18H,4-7,9,15-16H2,1-3H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253844
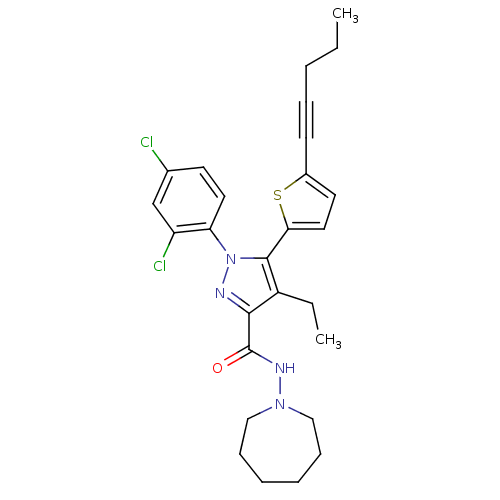
(CHEMBL460987 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...)Show SMILES CCCC#Cc1ccc(s1)-c1c(CC)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C27H30Cl2N4OS/c1-3-5-8-11-20-13-15-24(35-20)26-21(4-2)25(27(34)31-32-16-9-6-7-10-17-32)30-33(26)23-14-12-19(28)18-22(23)29/h12-15,18H,3-7,9-10,16-17H2,1-2H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253806
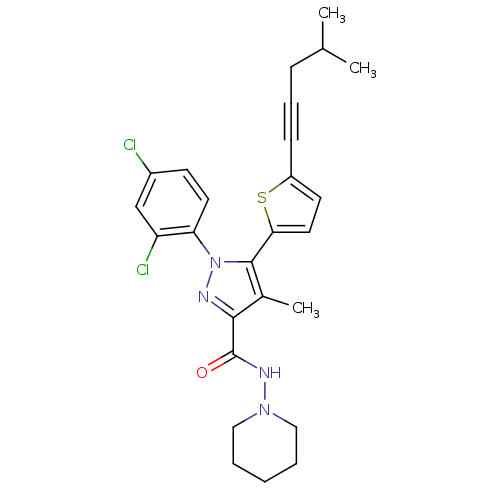
(1-(2,4-Dichlorophenyl)-4-methyl-5-(5-(4-methylpent...)Show SMILES CC(C)CC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H28Cl2N4OS/c1-17(2)8-7-9-20-11-13-23(34-20)25-18(3)24(26(33)30-31-14-5-4-6-15-31)29-32(25)22-12-10-19(27)16-21(22)28/h10-13,16-17H,4-6,8,14-15H2,1-3H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253805
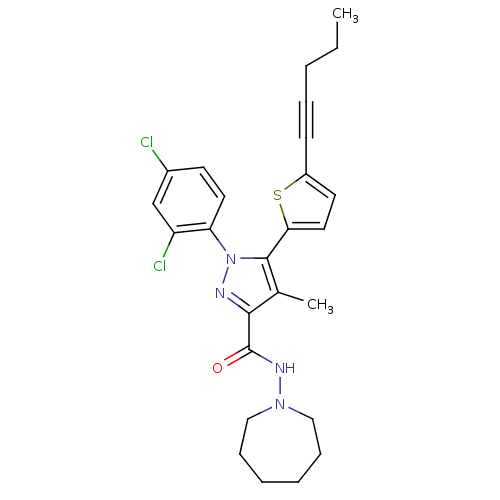
(CHEMBL460132 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...)Show SMILES CCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C26H28Cl2N4OS/c1-3-4-7-10-20-12-14-23(34-20)25-18(2)24(26(33)30-31-15-8-5-6-9-16-31)29-32(25)22-13-11-19(27)17-21(22)28/h11-14,17H,3-6,8-9,15-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253918
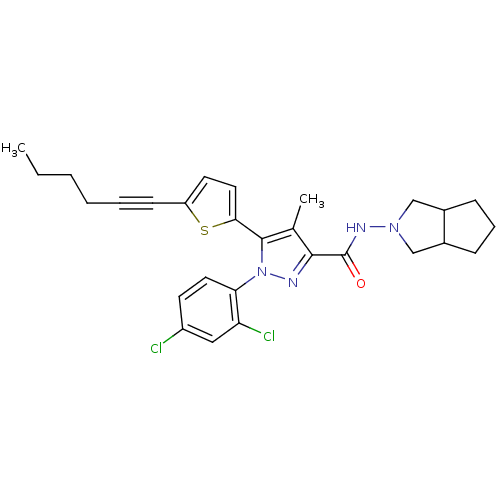
(1-(2,4-Dichlorophenyl)-5-(5-(hex-1-ynyl)thiophen-2...)Show SMILES CCCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CC2CCCC2C1 Show InChI InChI=1S/C28H30Cl2N4OS/c1-3-4-5-6-10-22-12-14-25(36-22)27-18(2)26(31-34(27)24-13-11-21(29)15-23(24)30)28(35)32-33-16-19-8-7-9-20(19)17-33/h11-15,19-20H,3-5,7-9,16-17H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253917
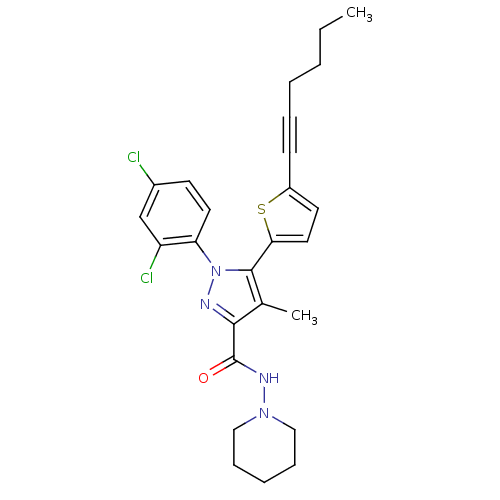
(1-(2,4-Dichlorophenyl)-5-(5-(hex-1-ynyl)thiophen-2...)Show SMILES CCCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H28Cl2N4OS/c1-3-4-5-7-10-20-12-14-23(34-20)25-18(2)24(26(33)30-31-15-8-6-9-16-31)29-32(25)22-13-11-19(27)17-21(22)28/h11-14,17H,3-6,8-9,15-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253298
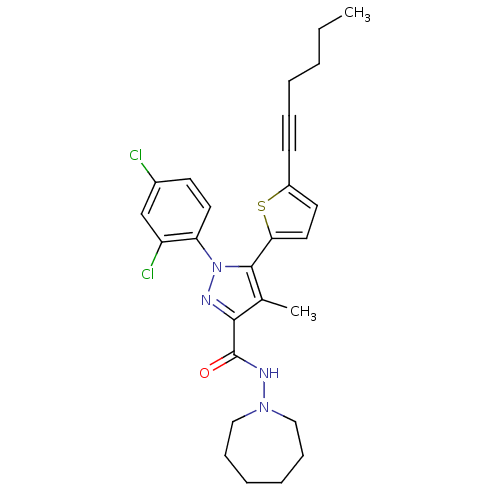
(CHEMBL522522 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...)Show SMILES CCCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C27H30Cl2N4OS/c1-3-4-5-8-11-21-13-15-24(35-21)26-19(2)25(27(34)31-32-16-9-6-7-10-17-32)30-33(26)23-14-12-20(28)18-22(23)29/h12-15,18H,3-7,9-10,16-17H2,1-2H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12178
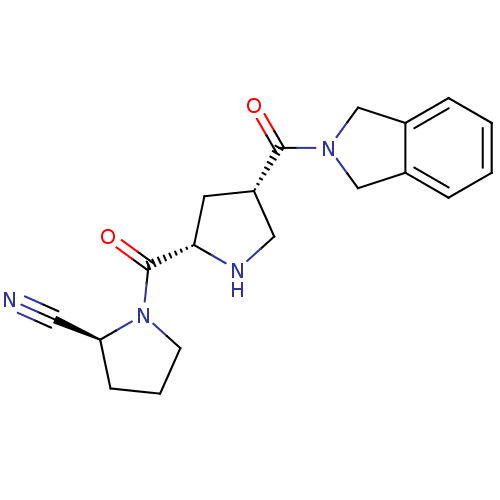
((2S)-1-{[(2S,4S)-4-(2,3-dihydro-1H-isoindol-2-ylca...)Show SMILES O=C([C@@H]1CN[C@@H](C1)C(=O)N1CCC[C@H]1C#N)N1Cc2ccccc2C1 |r| Show InChI InChI=1S/C19H22N4O2/c20-9-16-6-3-7-23(16)19(25)17-8-15(10-21-17)18(24)22-11-13-4-1-2-5-14(13)12-22/h1-2,4-5,15-17,21H,3,6-8,10-12H2/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes
| Assay Description
The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... |
Bioorg Med Chem Lett 16: 3268-72 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.037
BindingDB Entry DOI: 10.7270/Q22805V8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167698

(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50257317

((S)-1-((S)-2-amino-5-(isoindolin-2-yl)-5-oxopentan...)Show SMILES N[C@@H](CCC(=O)N1Cc2ccccc2C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H22N4O2/c19-10-15-6-3-9-22(15)18(24)16(20)7-8-17(23)21-11-13-4-1-2-5-14(13)12-21/h1-2,4-5,15-16H,3,6-9,11-12,20H2/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 19: 1908-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.061
BindingDB Entry DOI: 10.7270/Q2348K74 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434621
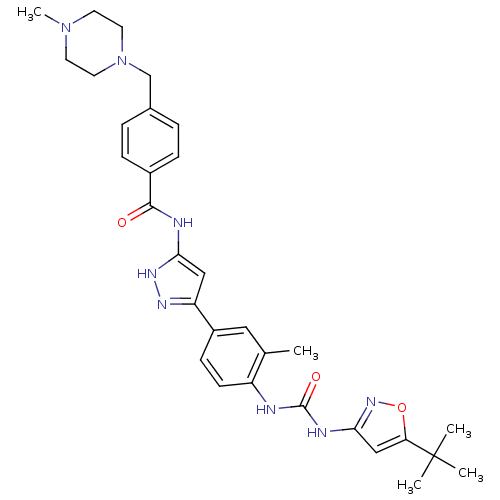
(CHEMBL2386796)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(NC(=O)Nc3cc(on3)C(C)(C)C)c(C)c2)CC1 Show InChI InChI=1S/C31H38N8O3/c1-20-16-23(10-11-24(20)32-30(41)34-28-18-26(42-37-28)31(2,3)4)25-17-27(36-35-25)33-29(40)22-8-6-21(7-9-22)19-39-14-12-38(5)13-15-39/h6-11,16-18H,12-15,19H2,1-5H3,(H2,32,34,37,41)(H2,33,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253300
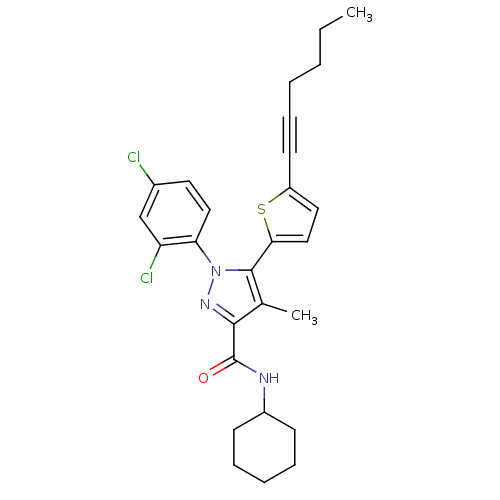
(CHEMBL494300 | N-Cyclohexyl-1-(2,4-dichlorophenyl)...)Show SMILES CCCCC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C27H29Cl2N3OS/c1-3-4-5-9-12-21-14-16-24(34-21)26-18(2)25(27(33)30-20-10-7-6-8-11-20)31-32(26)23-15-13-19(28)17-22(23)29/h13-17,20H,3-8,10-11H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253806

(1-(2,4-Dichlorophenyl)-4-methyl-5-(5-(4-methylpent...)Show SMILES CC(C)CC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H28Cl2N4OS/c1-17(2)8-7-9-20-11-13-23(34-20)25-18(3)24(26(33)30-31-14-5-4-6-15-31)29-32(25)22-12-10-19(27)16-21(22)28/h10-13,16-17H,4-6,8,14-15H2,1-3H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50265158

(CHEMBL497992 | N-((trans)-4-((S)-1-amino-2-oxo-2-(...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(NS(=O)(=O)CC(F)(F)F)cc1)C(=O)N1CCCC1 |r,wU:5.8,wD:2.1,1.0,(13.27,5.92,;14.6,5.15,;14.6,3.61,;13.27,2.83,;13.27,1.28,;14.6,.51,;15.95,1.28,;15.95,2.83,;14.6,-1.03,;13.27,-1.79,;14.03,-3.13,;12.5,-.46,;11.93,-2.55,;10.6,-1.76,;9.26,-2.51,;9.25,-4.05,;7.91,-4.81,;7.89,-6.35,;6.35,-6.34,;9.43,-6.37,;7.88,-7.89,;6.54,-8.65,;5.2,-9.4,;7.3,-9.99,;5.78,-7.31,;10.58,-4.84,;11.91,-4.08,;15.93,5.92,;15.93,7.46,;17.27,5.15,;17.43,3.63,;18.93,3.31,;19.7,4.64,;18.67,5.79,)| Show InChI InChI=1S/C20H29F3N4O5S2/c21-20(22,23)13-33(29,30)25-15-7-9-17(10-8-15)34(31,32)26-16-5-3-14(4-6-16)18(24)19(28)27-11-1-2-12-27/h7-10,14,16,18,25-26H,1-6,11-13,24H2/t14-,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus system |
Eur J Med Chem 43: 1603-11 (2008)
Article DOI: 10.1016/j.ejmech.2007.11.014
BindingDB Entry DOI: 10.7270/Q2V69JCD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253299
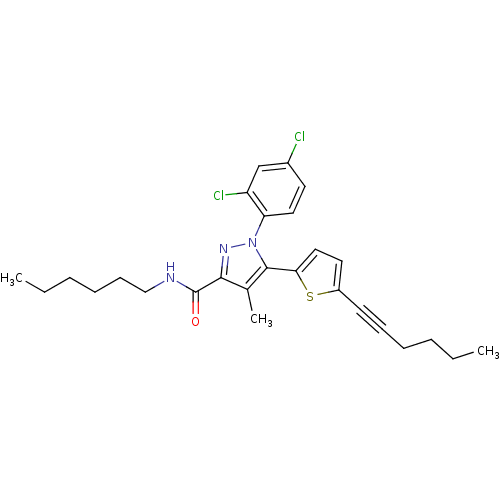
(1-(2,4-Dichlorophenyl)-5-(5-(hex-1-ynyl)thiophen-2...)Show SMILES CCCCCCNC(=O)c1nn(c(c1C)-c1ccc(s1)C#CCCCC)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H31Cl2N3OS/c1-4-6-8-10-12-21-14-16-24(34-21)26-19(3)25(27(33)30-17-11-9-7-5-2)31-32(26)23-15-13-20(28)18-22(23)29/h13-16,18H,4-9,11,17H2,1-3H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253757
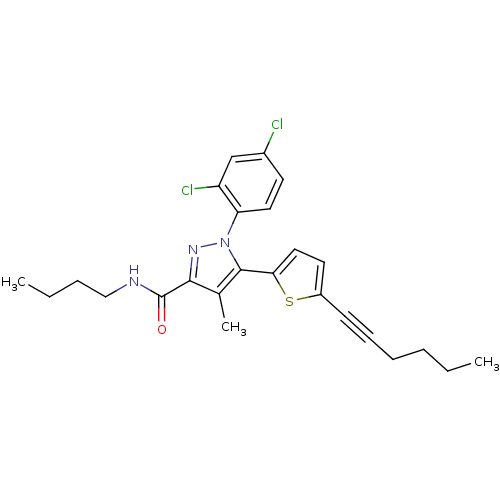
(CHEMBL462069 | N-Butyl-1-(2,4-dichlorophenyl)-5-(5...)Show SMILES CCCCNC(=O)c1nn(c(c1C)-c1ccc(s1)C#CCCCC)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H27Cl2N3OS/c1-4-6-8-9-10-19-12-14-22(32-19)24-17(3)23(25(31)28-15-7-5-2)29-30(24)21-13-11-18(26)16-20(21)27/h11-14,16H,4-8,15H2,1-3H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged VEGFR2 expressed in Sf9 insect cells after 120 mins by Kinase-Glo assay |
Bioorg Med Chem 19: 4173-82 (2011)
Article DOI: 10.1016/j.bmc.2011.06.016
BindingDB Entry DOI: 10.7270/Q2CR5TQV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3596-600 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.124
BindingDB Entry DOI: 10.7270/Q29Z953W |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389222
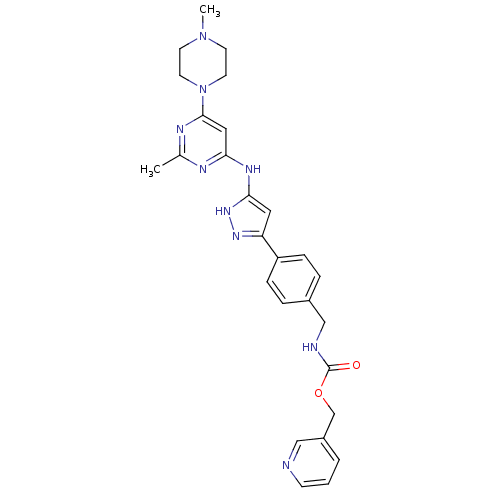
(CHEMBL2063336)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cccnc3)cc2)nc(C)n1 Show InChI InChI=1S/C27H31N9O2/c1-19-30-24(15-26(31-19)36-12-10-35(2)11-13-36)32-25-14-23(33-34-25)22-7-5-20(6-8-22)17-29-27(37)38-18-21-4-3-9-28-16-21/h3-9,14-16H,10-13,17-18H2,1-2H3,(H,29,37)(H2,30,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434614
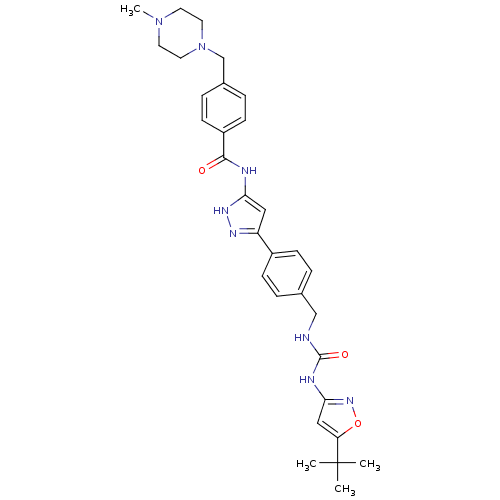
(CHEMBL2386803)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(CNC(=O)Nc3cc(on3)C(C)(C)C)cc2)CC1 Show InChI InChI=1S/C31H38N8O3/c1-31(2,3)26-18-28(37-42-26)34-30(41)32-19-21-5-9-23(10-6-21)25-17-27(36-35-25)33-29(40)24-11-7-22(8-12-24)20-39-15-13-38(4)14-16-39/h5-12,17-18H,13-16,19-20H2,1-4H3,(H2,32,34,37,41)(H2,33,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis |
Bioorg Med Chem 21: 2856-67 (2013)
Article DOI: 10.1016/j.bmc.2013.03.083
BindingDB Entry DOI: 10.7270/Q22N53NF |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253807

(CHEMBL462686 | N-(Azepan-1-yl)-1-(2,4-dichlorophen...)Show SMILES CC(C)CC#Cc1ccc(s1)-c1c(C)c(nn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCCC1 Show InChI InChI=1S/C27H30Cl2N4OS/c1-18(2)9-8-10-21-12-14-24(35-21)26-19(3)25(27(34)31-32-15-6-4-5-7-16-32)30-33(26)23-13-11-20(28)17-22(23)29/h11-14,17-18H,4-7,9,15-16H2,1-3H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 5397-412 (2008)
Article DOI: 10.1021/jm800066v
BindingDB Entry DOI: 10.7270/Q24M94BM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320120
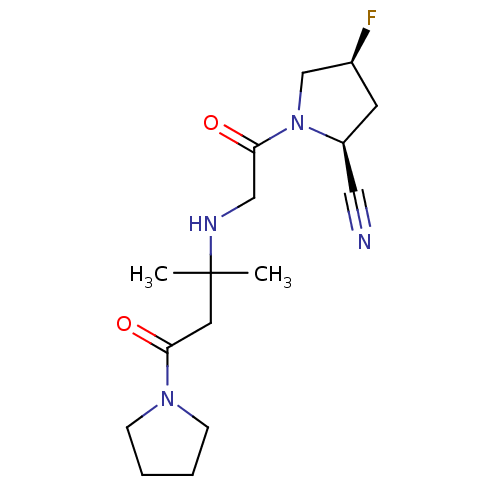
((2S,4S)-4-fluoro-1-(2-(2-methyl-4-oxo-4-(pyrrolidi...)Show SMILES CC(C)(CC(=O)N1CCCC1)NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C16H25FN4O2/c1-16(2,8-14(22)20-5-3-4-6-20)19-10-15(23)21-11-12(17)7-13(21)9-18/h12-13,19H,3-8,10-11H2,1-2H3/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in presence of 50% human serum |
Bioorg Med Chem Lett 20: 3596-600 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.124
BindingDB Entry DOI: 10.7270/Q29Z953W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins by H... |
Bioorg Med Chem 22: 4694-703 (2014)
Article DOI: 10.1016/j.bmc.2014.07.012
BindingDB Entry DOI: 10.7270/Q2H133NH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data