

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582018 (US11518754, Compound (R)-3) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582020 (US11518754, Compound (R)-5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582019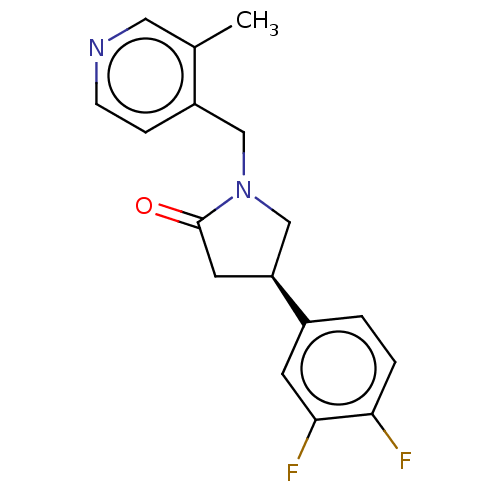 (US11518754, Compound (R)-4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582022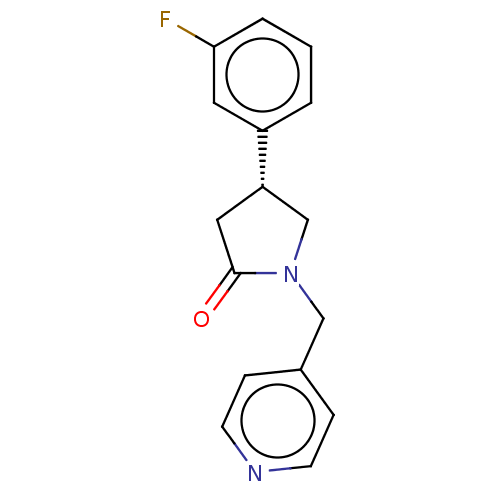 (US11518754, Compound (R)-12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582021 (US11518754, Compound (R)-11) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582027 (US11518754, Compound (S)-12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582024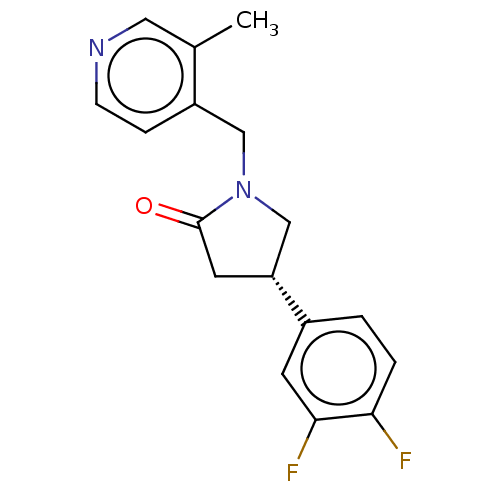 (US11518754, Compound (S)-4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50174316 (CHEMBL3809355) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry | ACS Med Chem Lett 7: 424-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00009 BindingDB Entry DOI: 10.7270/Q2BZ680Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582023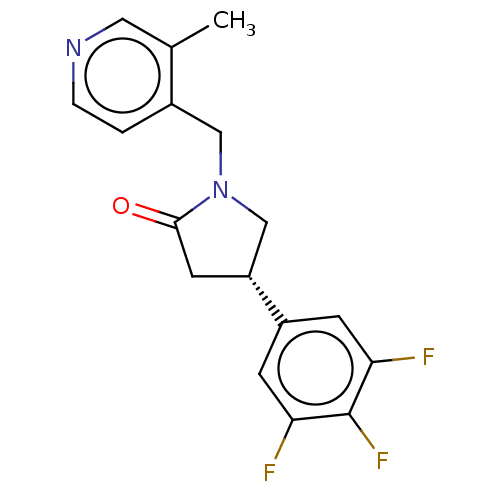 (US11518754, Compound (S)-3) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582026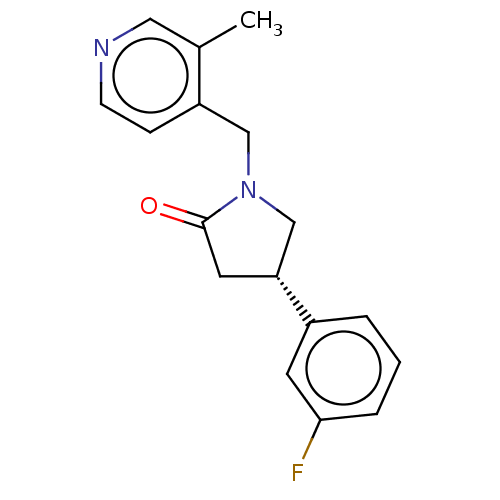 (US11518754, Compound (S)-11) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50149232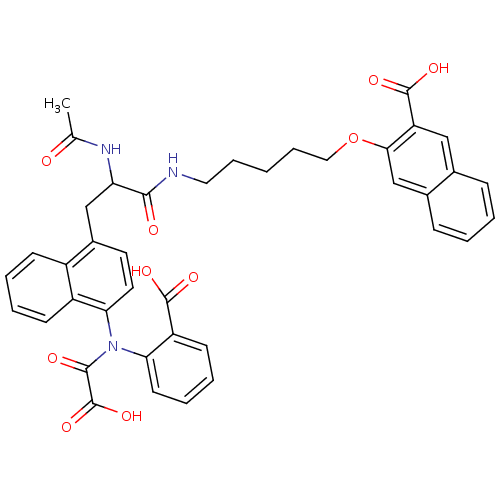 (3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Bioorg Med Chem 16: 7399-409 (2008) Article DOI: 10.1016/j.bmc.2008.06.014 BindingDB Entry DOI: 10.7270/Q2PC325T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Mus musculus) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Flk1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50092588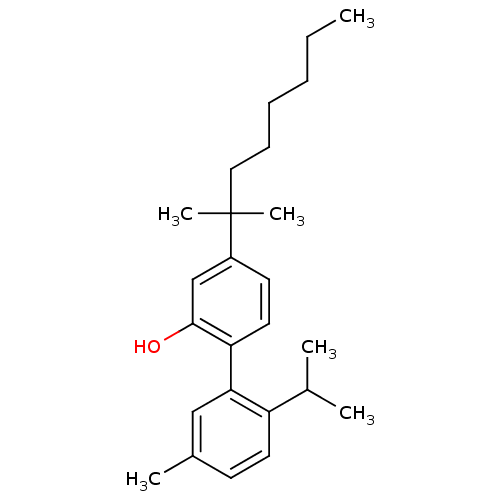 (4-(1,1-Dimethyl-heptyl)-2'-isopropyl-5'-methyl-bip...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry | ACS Med Chem Lett 7: 424-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00009 BindingDB Entry DOI: 10.7270/Q2BZ680Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50149232 (3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of TCPTP (unknown origin) | Bioorg Med Chem 16: 7399-409 (2008) Article DOI: 10.1016/j.bmc.2008.06.014 BindingDB Entry DOI: 10.7270/Q2PC325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582025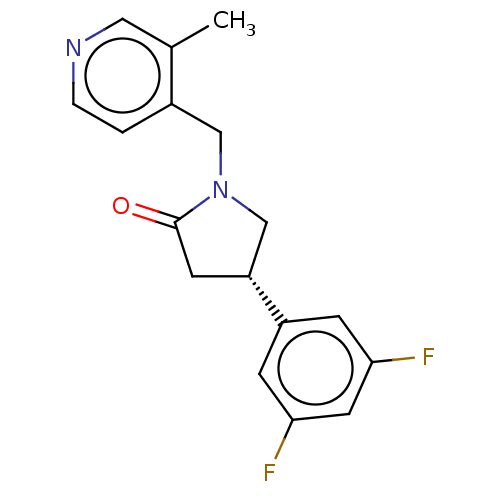 (US11518754, Compound (S)-5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50174315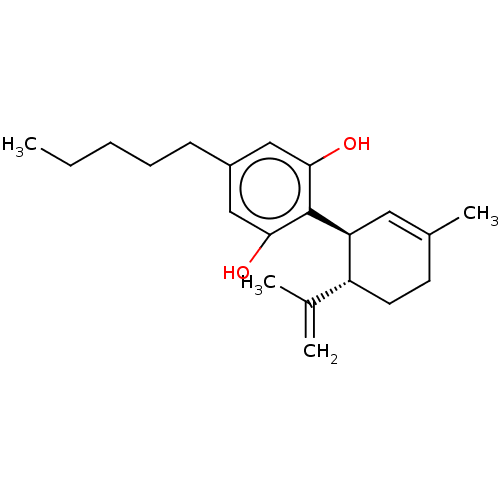 (CHEMBL3810140) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins | ACS Med Chem Lett 7: 424-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00009 BindingDB Entry DOI: 10.7270/Q2BZ680Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM50149232 (3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of LAR (unknown origin) | Bioorg Med Chem 16: 7399-409 (2008) Article DOI: 10.1016/j.bmc.2008.06.014 BindingDB Entry DOI: 10.7270/Q2PC325T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins | ACS Med Chem Lett 7: 424-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00009 BindingDB Entry DOI: 10.7270/Q2BZ680Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50021574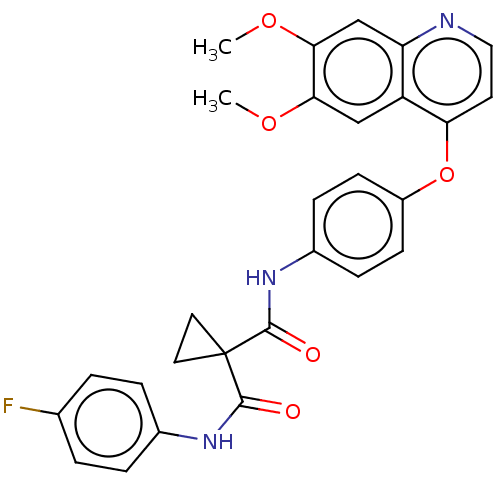 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of VEGFR-2 (unknown origin) | Bioorg Med Chem 25: 3195-3205 (2017) Article DOI: 10.1016/j.bmc.2017.04.003 BindingDB Entry DOI: 10.7270/Q27P91JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50168389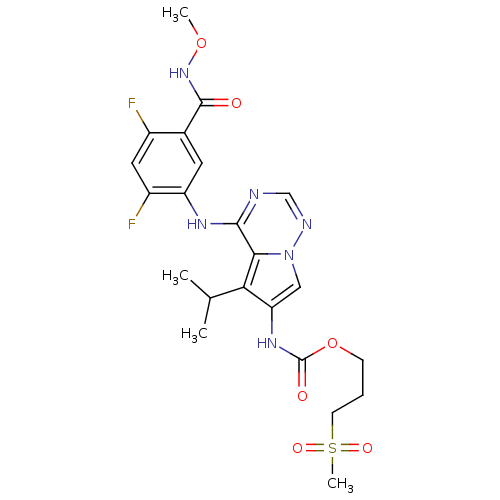 (CHEMBL195218 | [4-(2,4-Difluoro-5-methoxycarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human vascular endothelial growth factor receptor 2 (VEGFR-2) | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50021574 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of VEGFR-2 (unknown origin) by HTRF method | Bioorg Med Chem 25: 3195-3205 (2017) Article DOI: 10.1016/j.bmc.2017.04.003 BindingDB Entry DOI: 10.7270/Q27P91JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279989 (US10028961, Compound 142 | US10172864, Compound 14...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279977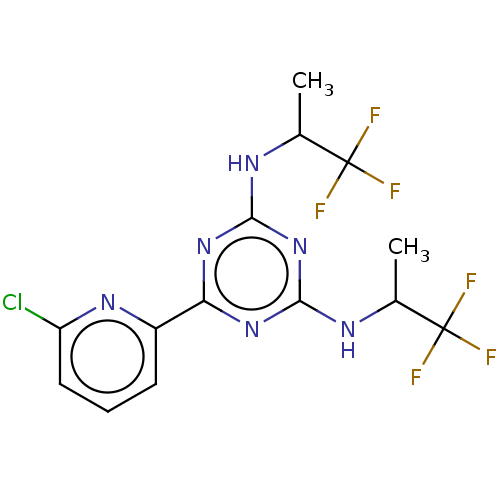 (US10028961, Compound 130 | US10172864, Compound 13...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha (unknown origin) by HTRF method | Bioorg Med Chem 25: 3195-3205 (2017) Article DOI: 10.1016/j.bmc.2017.04.003 BindingDB Entry DOI: 10.7270/Q27P91JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of FLT3 (unknown origin) by HTRF method | Bioorg Med Chem 25: 3195-3205 (2017) Article DOI: 10.1016/j.bmc.2017.04.003 BindingDB Entry DOI: 10.7270/Q27P91JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50612938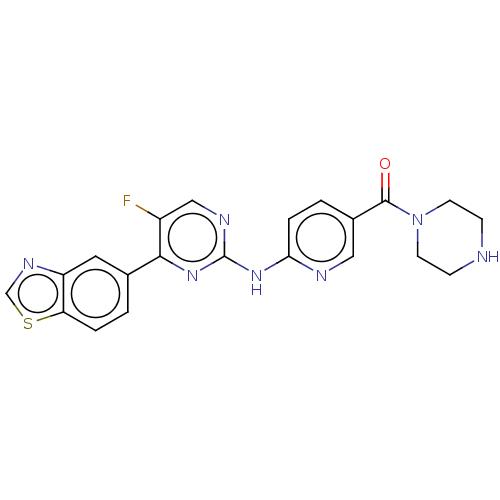 (CHEMBL5289179) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279960 (US10028961, Compound 113 | US10172864, Compound 11...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279996 (US10028961, Compound 149 | US10172864, Compound 14...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50465394 (CHEMBL4278793) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132C mutant in human HT1080 cells assessed as reduction in 2-hydroxyglutarate production by LC-MS/MS analysis | ACS Med Chem Lett 9: 300-305 (2018) Article DOI: 10.1021/acsmedchemlett.7b00421 BindingDB Entry DOI: 10.7270/Q2NC63WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28028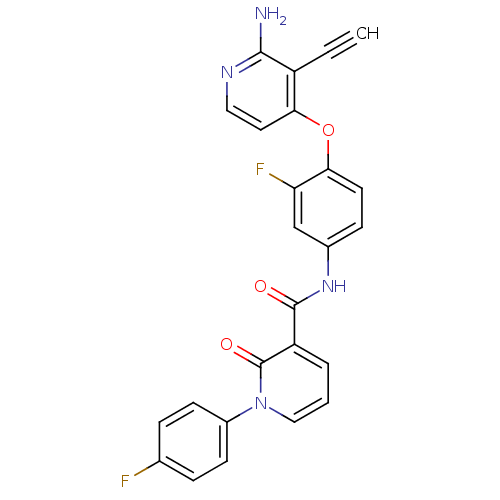 (2-aminopyridine analogue, 7 | N-{4-[(2-amino-3-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... | J Med Chem 52: 1251-4 (2009) Article DOI: 10.1021/jm801586s BindingDB Entry DOI: 10.7270/Q20863MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28030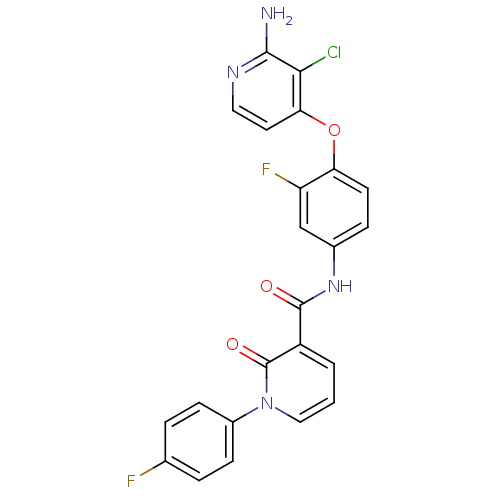 (2-aminopyridine analogue, 9 | N-{4-[(2-amino-3-chl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... | J Med Chem 52: 1251-4 (2009) Article DOI: 10.1021/jm801586s BindingDB Entry DOI: 10.7270/Q20863MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1/2/3/4 (Homo sapiens (Human)) | BDBM50168394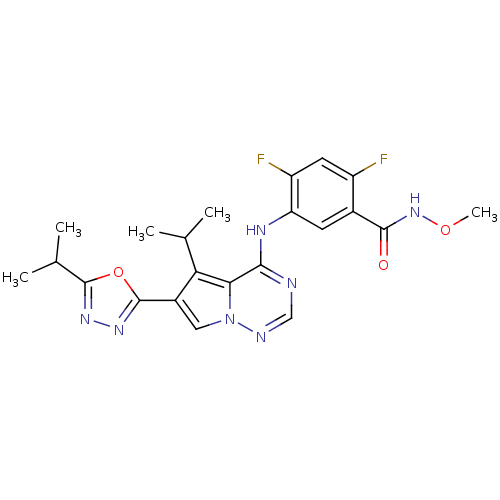 (2,4-Difluoro-5-[5-isopropyl-6-(5-isopropyl-[1,3,4]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of FGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279884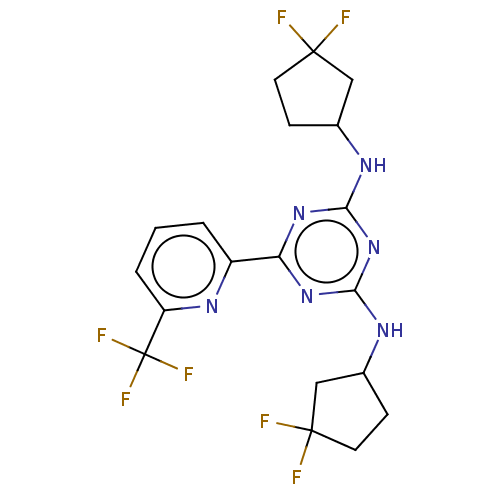 (US10028961, Compound 31 | US10172864, Compound 31 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50465396 (CHEMBL4289465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132C mutant in human HT1080 cells assessed as reduction in 2-hydroxyglutarate production by LC-MS/MS analysis | ACS Med Chem Lett 9: 300-305 (2018) Article DOI: 10.1021/acsmedchemlett.7b00421 BindingDB Entry DOI: 10.7270/Q2NC63WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1/2/3 (Homo sapiens (Human)) | BDBM50168397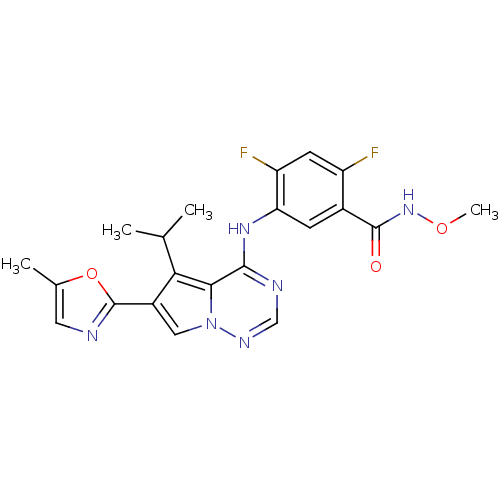 (2,4-Difluoro-5-[5-isopropyl-6-(5-methyl-oxazol-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of VEGF-stimulated human umbilical vein endothelial cell proliferation | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24457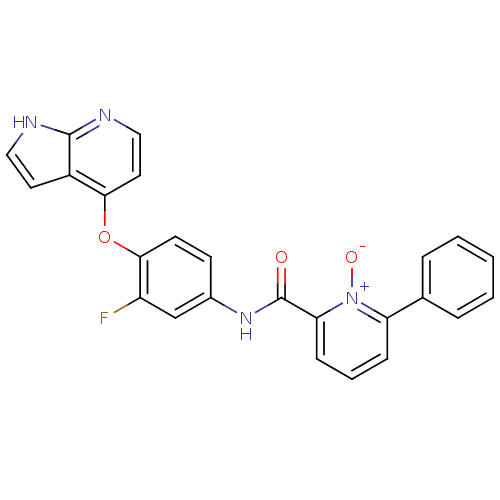 (2-[(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company | Assay Description Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... | J Med Chem 51: 5330-41 (2008) Article DOI: 10.1021/jm800476q BindingDB Entry DOI: 10.7270/Q2K35RZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50021574 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of c-MET (unknown origin) | Bioorg Med Chem 25: 3195-3205 (2017) Article DOI: 10.1016/j.bmc.2017.04.003 BindingDB Entry DOI: 10.7270/Q27P91JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50235550 (1-(4-(5-((4-aminocyclohexylidene)methyl)pyrrolo[1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system | Bioorg Med Chem Lett 18: 1945-51 (2008) Article DOI: 10.1016/j.bmcl.2008.01.121 BindingDB Entry DOI: 10.7270/Q2125SFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582018 (US11518754, Compound (R)-3) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460918 (US10774053, Compound 32 | US11352329, COMPD # 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460922 (US10774053, Compound 36 | US11352329, COMPD # 36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460924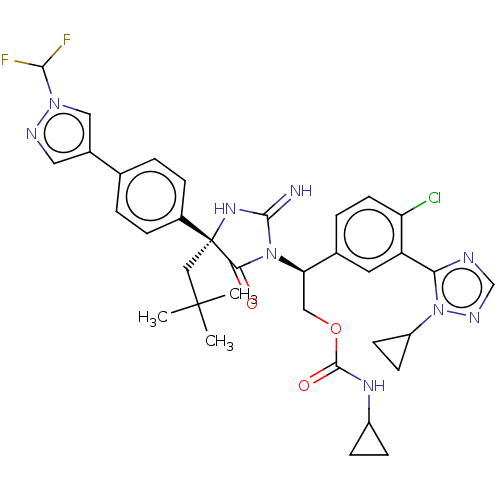 (US10774053, Compound 38 | US11352329, COMPD # 38) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460925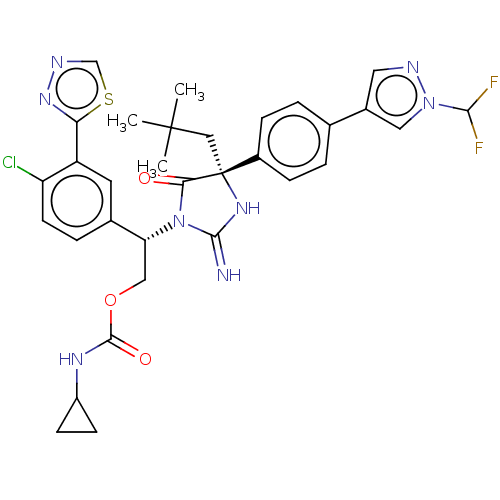 (US10774053, Compound 39 | US11352329, COMPD # 39) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460930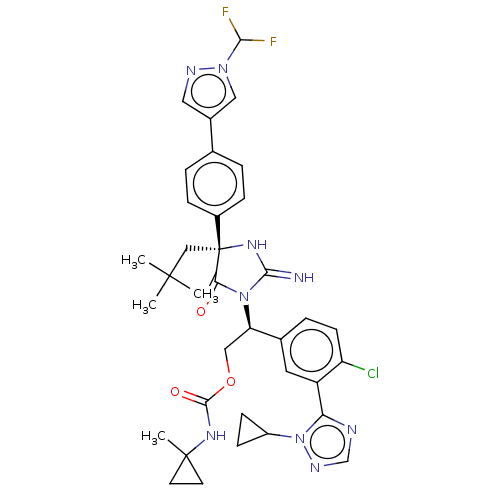 (US10774053, Compound 43 | US11352329, COMPD # 43) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460932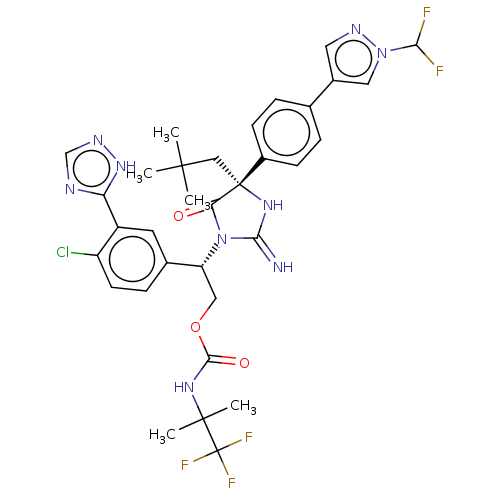 (US10774053, Compound 45 | US11352329, COMPD # 45) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460936 (US10774053, Compound 49 | US11352329, COMPD # 49) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (HIV-1) | BDBM460965 (US10774053, Compound 85 | US11352329, COMPD # 85) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibitor potency against HIV protease was measured using an enzymatic assay with a fluorogenic readout. To a reaction buffer containing 100 mM ammon... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8679 total ) | Next | Last >> |