

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (GUINEA PIG) | BDBM50071342 (4-Chloro-N-[4-(1H-imidazol-4-yl)-butyl]-N-(4-imida...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071343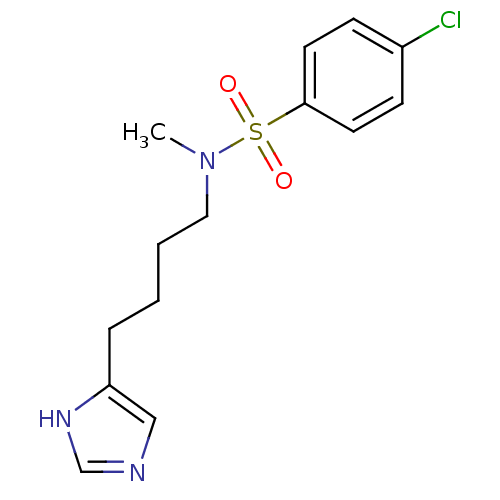 (4-Chloro-N-[4-(1H-imidazol-4-yl)-butyl]-N-methyl-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071348 (4-tert-Butyl-N-[5-(1H-imidazol-4-yl)-pentyl]-benze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071340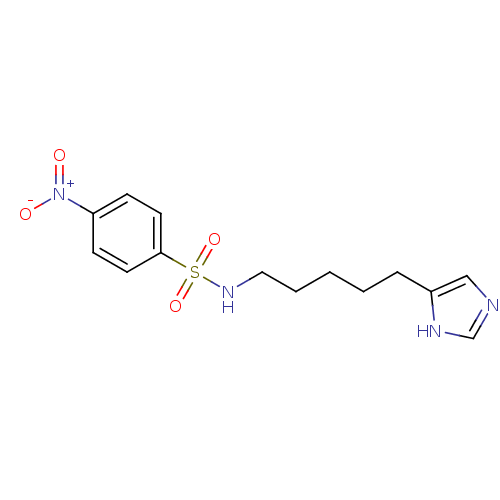 (CHEMBL68336 | N-[5-(1H-Imidazol-4-yl)-pentyl]-4-ni...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071352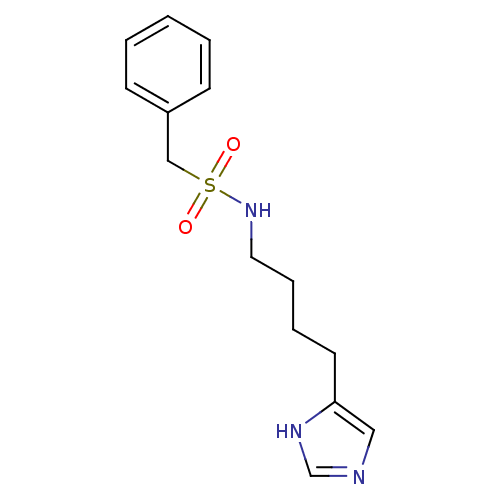 (CHEMBL69594 | N-[4-(1H-Imidazol-4-yl)-butyl]-C-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071339 (CHEMBL68475 | N-[4-(1H-Imidazol-4-yl)-butyl]-4-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071358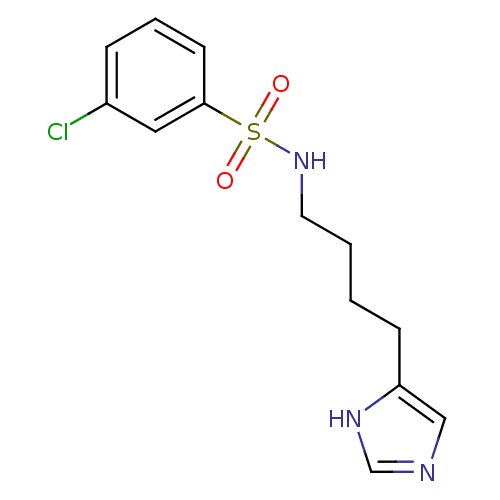 (3-Chloro-N-[4-(1H-imidazol-4-yl)-butyl]-benzenesul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071346 (4-Chloro-N-[4-(1H-imidazol-4-yl)-butyl]-benzenesul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071344 (CHEMBL71592 | N-[4-(1H-Imidazol-4-yl)-butyl]-4-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071354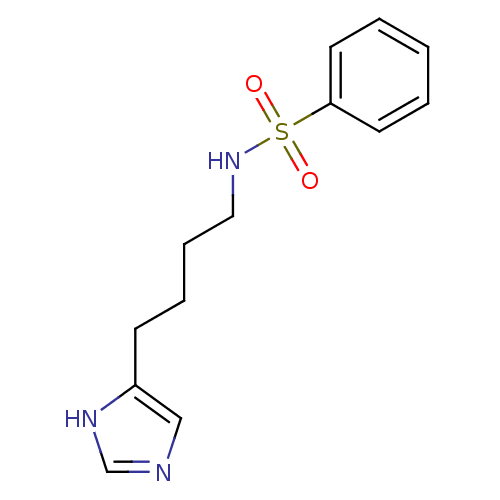 (CHEMBL69551 | N-[4-(1H-Imidazol-4-yl)-butyl]-benze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071336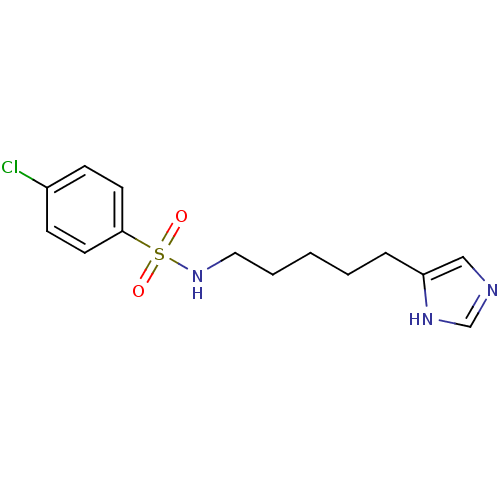 (4-Chloro-N-[5-(1H-imidazol-4-yl)-pentyl]-benzenesu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071351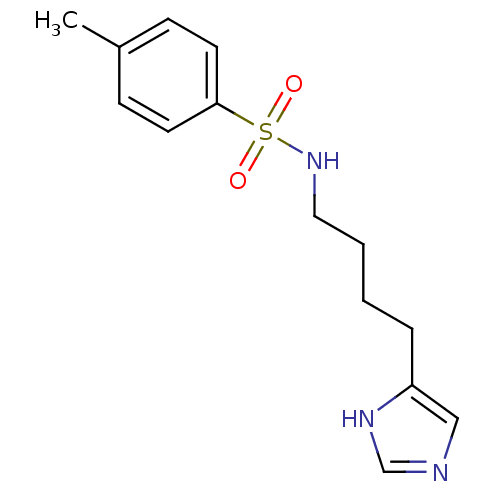 (CHEMBL69456 | N-[4-(1H-Imidazol-4-yl)-butyl]-4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071337 (CHEMBL69607 | N-[4-(1H-Imidazol-4-yl)-butyl]-4-nit...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071356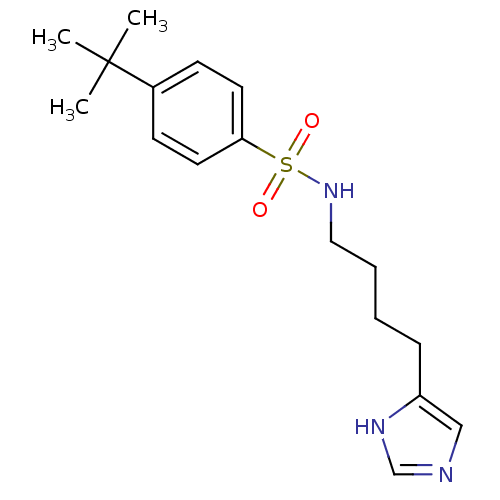 (4-tert-Butyl-N-[4-(1H-imidazol-4-yl)-butyl]-benzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071347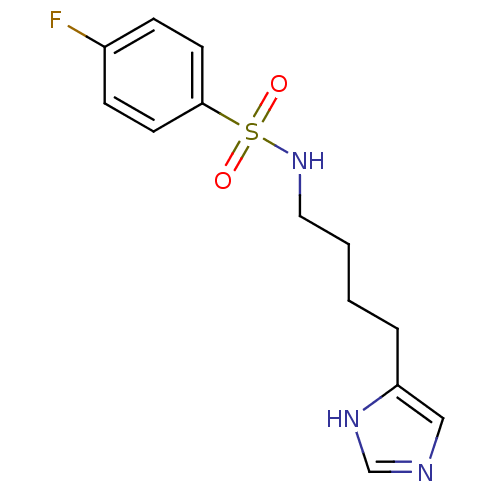 (4-Fluoro-N-[4-(1H-imidazol-4-yl)-butyl]-benzenesul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071338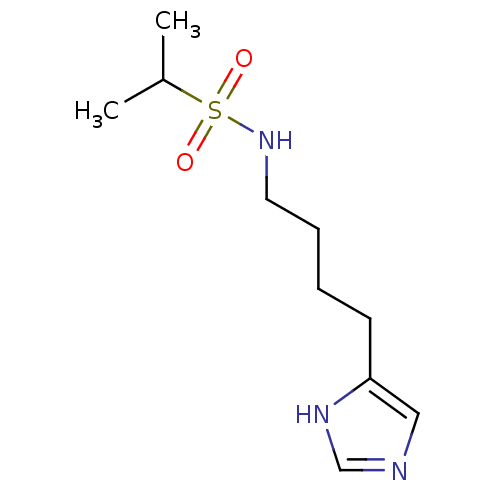 (CHEMBL66439 | Propane-2-sulfonic acid [4-(1H-imida...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071359 (4-Chloro-N-(4-chloro-benzyl)-N-[4-(1H-imidazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071361 (CHEMBL71941 | N-[5-(1H-Imidazol-4-yl)-pentyl]-4-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071355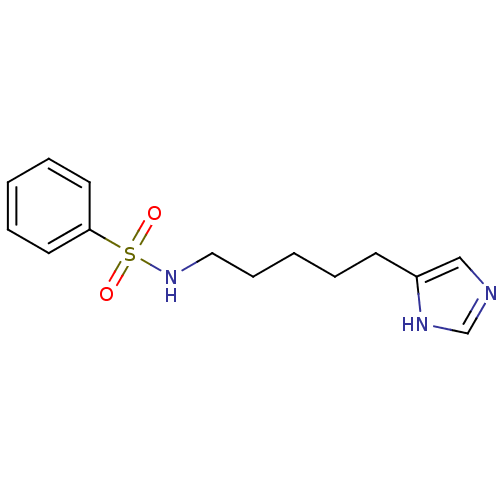 (CHEMBL69279 | N-[5-(1H-Imidazol-4-yl)-pentyl]-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071363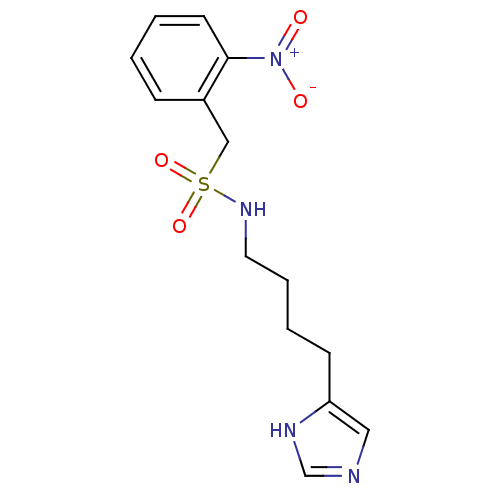 (CHEMBL69682 | N-[4-(1H-Imidazol-4-yl)-butyl]-C-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071350 (1-[4-(1H-Imidazol-4-yl)-butylsulfamoyl]-3-methyl-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071349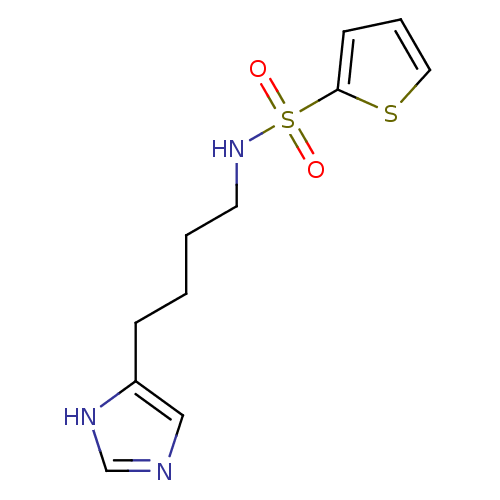 (CHEMBL305174 | Thiophene-2-sulfonic acid [4-(1H-im...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071353 (4-tert-Butyl-N-[3-(1H-imidazol-4-yl)-propyl]-benze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071362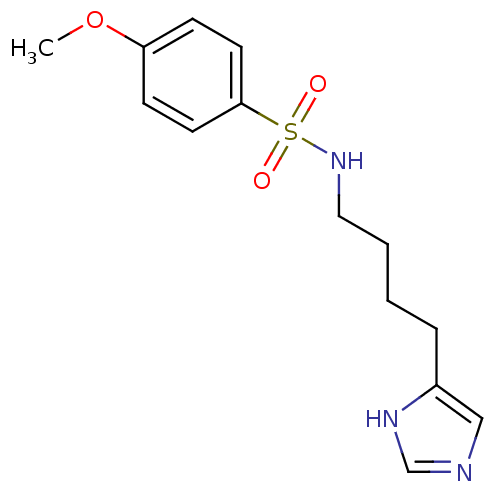 (CHEMBL69044 | N-[4-(1H-Imidazol-4-yl)-butyl]-4-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071357 (CHEMBL308899 | N-[3-(1H-Imidazol-4-yl)-propyl]-4-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071360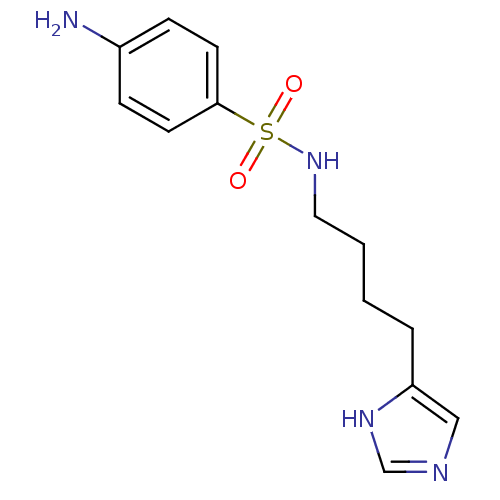 (4-Amino-N-[4-(1H-imidazol-4-yl)-butyl]-benzenesulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071345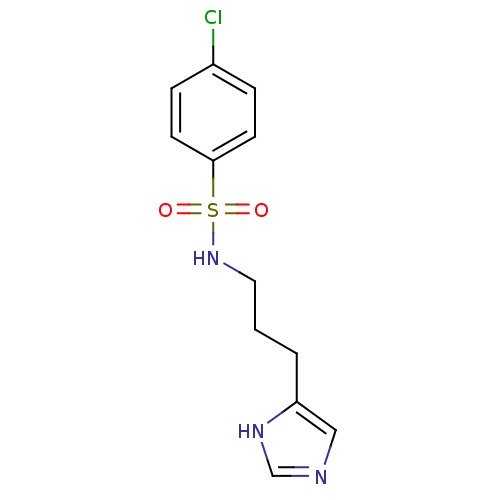 (4-Chloro-N-[3-(1H-imidazol-4-yl)-propyl]-benzenesu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071341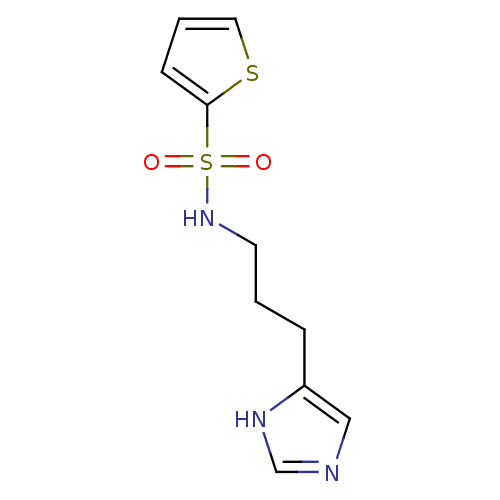 (CHEMBL69707 | Thiophene-2-sulfonic acid [3-(1H-imi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-Nalpha-methylhistamine binding to H3 receptor in guinea-pig brain membranes | Bioorg Med Chem Lett 8: 2157-62 (1999) BindingDB Entry DOI: 10.7270/Q23T9GCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079085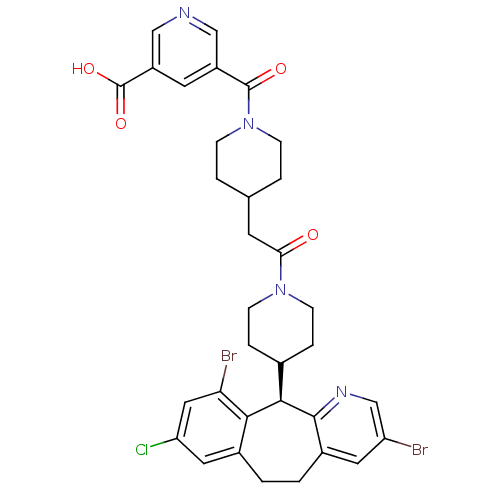 (5-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079090 (CHEMBL90435 | [(4-{2-[4-((R)-3,10-Dibromo-8-chloro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079075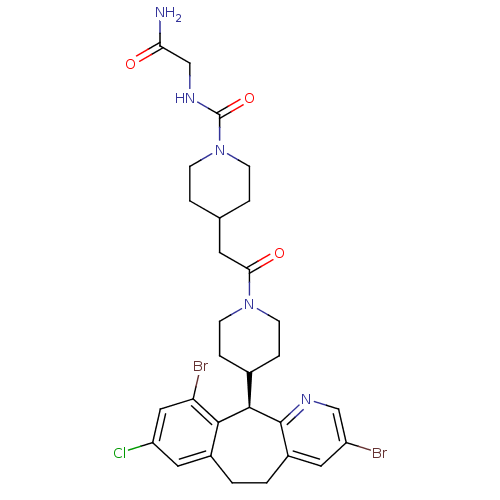 (4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into His6-H-Ras-CVLS by farnesyl transferase | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14457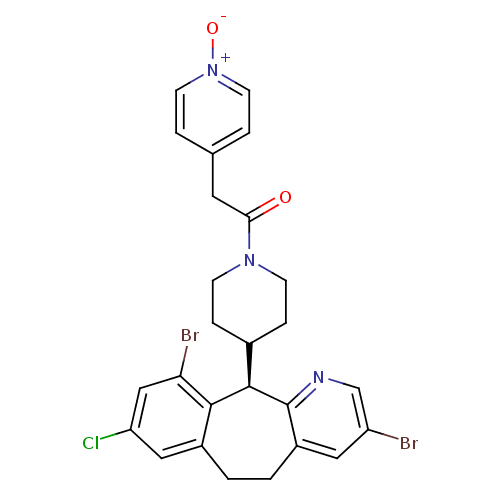 ((+)-4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079079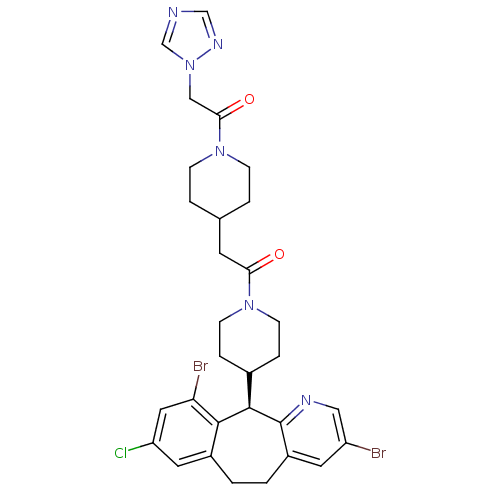 (1-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14461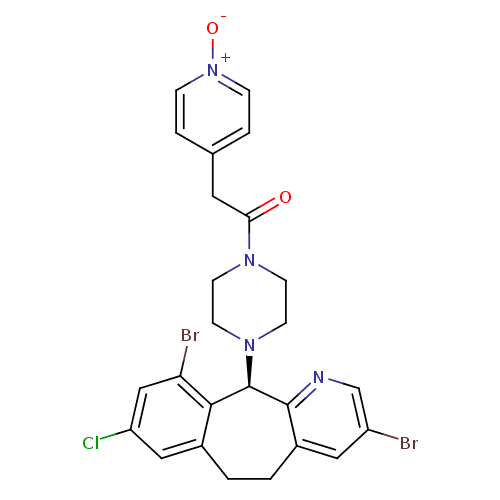 ((+)-4-(3,10-Dibromo-8-chloro-6,11-dihydro-5H-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM14459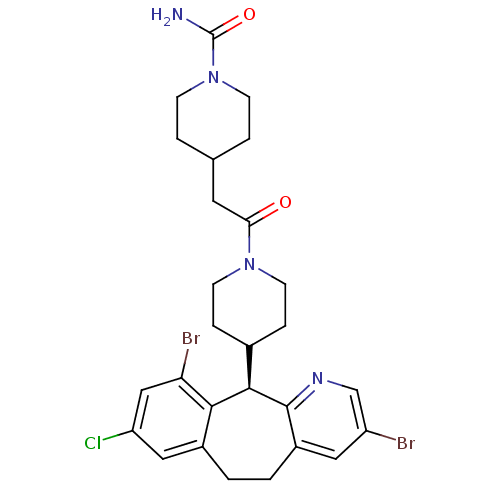 ((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14459 ((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079074 (4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079089 (1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079084 (CHEMBL432573 | [(4-{2-[4-((R)-3,10-Dibromo-8-chlor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079083 (1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Hras Farnesyl protein transferase(FPT) | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079073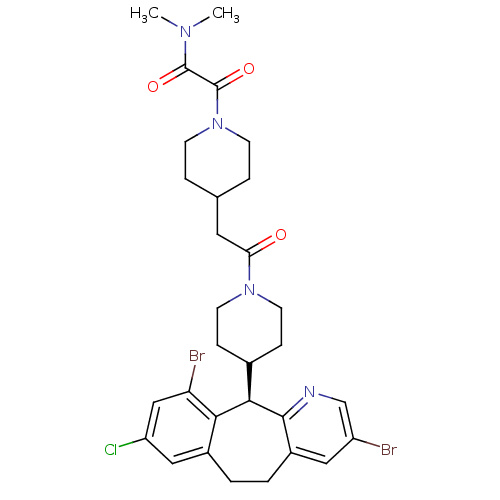 (2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079091 (2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into His6-H-Ras-CVLS by farnesyl transferase | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079076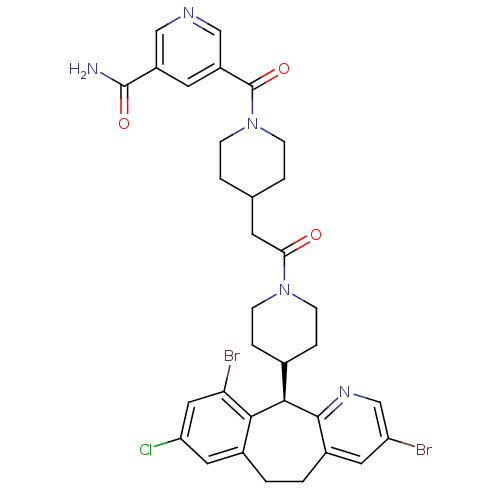 (5-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14473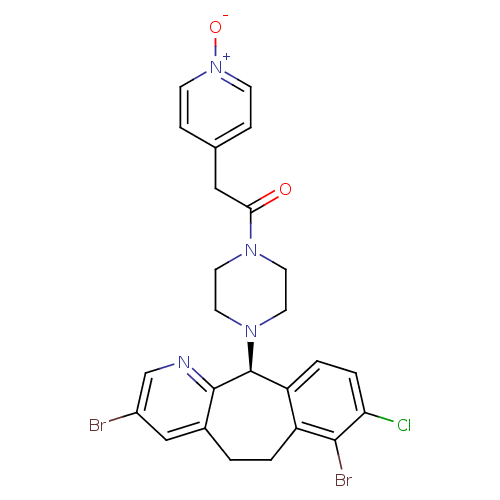 ((-)-1-(8-Chloro-3,7-dibromo-6,11-dihydro-5H-benzo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079070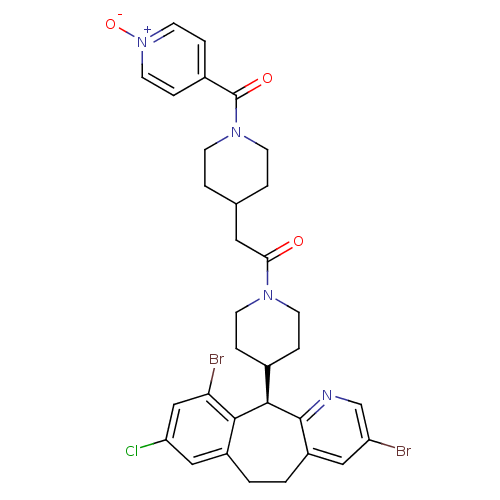 (1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Hras Farnesyl protein transferase(FPT) | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14467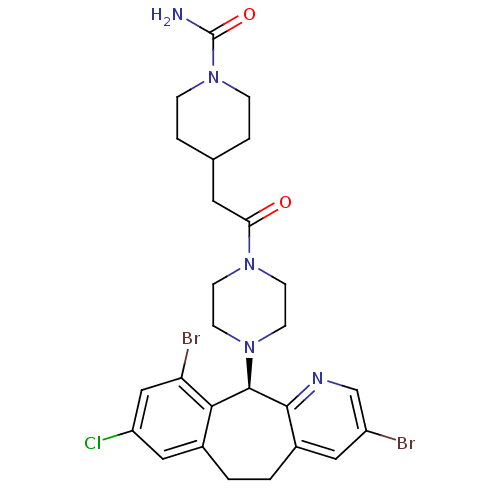 ((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14469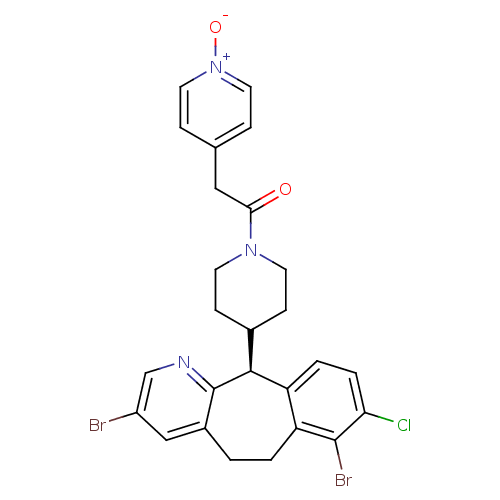 ((-)-4-(8-Chloro-3,7-dibromo-6,11-dihydro-5H-benzo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079086 (3-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Hras Farnesyl protein transferase(FPT) | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50079077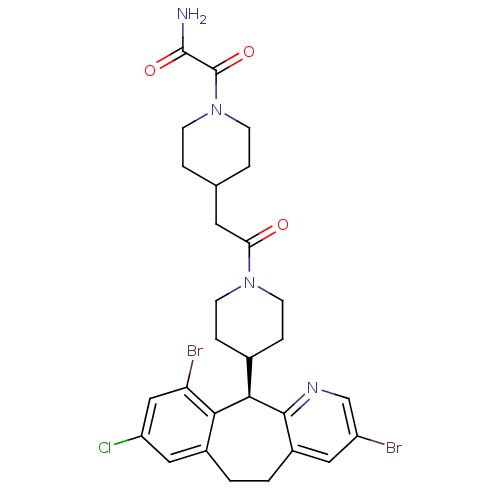 (2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Hras Farnesyltransferase (FPT). | J Med Chem 42: 2651-61 (1999) Article DOI: 10.1021/jm990059k BindingDB Entry DOI: 10.7270/Q2ST7P2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14477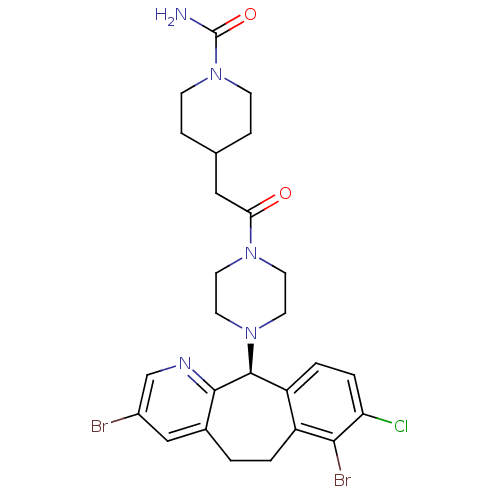 ((-)-4-[2-[4-(3,7-Dibromo-8-chloro-6,11-dihydro-5Hb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute | Assay Description FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... | J Med Chem 41: 4890-902 (1998) Article DOI: 10.1021/jm980462b BindingDB Entry DOI: 10.7270/Q2ZS2TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 162 total ) | Next | Last >> |