 Found 396 hits with Last Name = 'alexander' and Initial = 'j'
Found 396 hits with Last Name = 'alexander' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226932
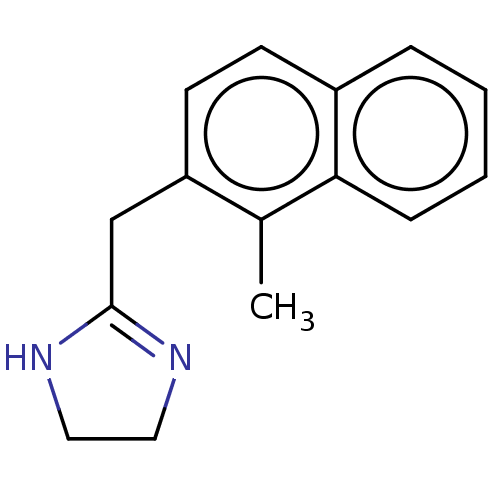
(CHEMBL559829)Show InChI InChI=1S/C15H16N2/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12/h2-7H,8-10H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226924
(CHEMBL545647)Show InChI InChI=1S/C14H16N2/c1-10-12(9-14-15-6-7-16-14)8-11-4-2-3-5-13(10)11/h2-5H,6-9H2,1H3,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50019848
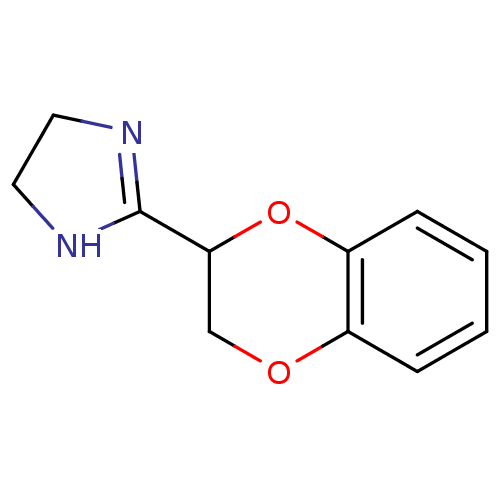
(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-clonidine from alpha-1 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226929

(CHEMBL545646)Show InChI InChI=1S/C15H18N2/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12/h2-5H,6-10H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226929

(CHEMBL545646)Show InChI InChI=1S/C15H18N2/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12/h2-5H,6-10H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50454831
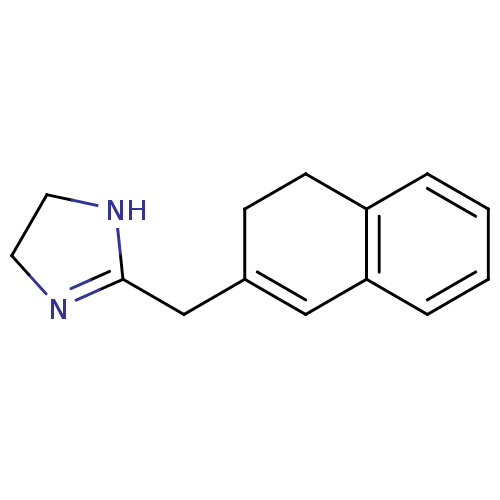
(Napamezole)Show InChI InChI=1S/C14H16N2/c1-2-4-13-9-11(5-6-12(13)3-1)10-14-15-7-8-16-14/h1-4,9H,5-8,10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50454831

(Napamezole)Show InChI InChI=1S/C14H16N2/c1-2-4-13-9-11(5-6-12(13)3-1)10-14-15-7-8-16-14/h1-4,9H,5-8,10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226928

(CHEMBL553439)Show InChI InChI=1S/C15H18N2.ClH/c1-11(15-16-8-9-17-15)13-7-6-12-4-2-3-5-14(12)10-13;/h2-5,10-11H,6-9H2,1H3,(H,16,17);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50057120

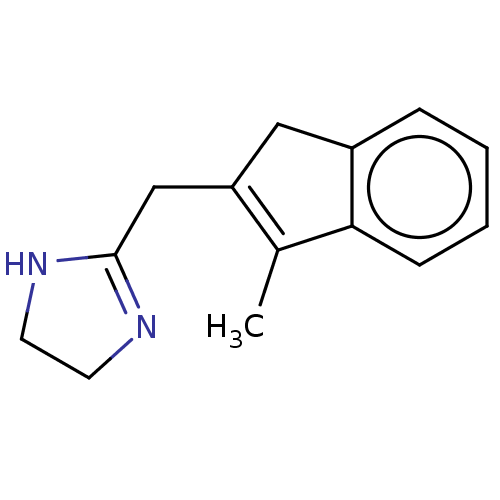
(2-Naphthalen-2-ylmethyl-4,5-dihydro-1H-imidazole |...)Show InChI InChI=1S/C14H14N2/c1-2-4-13-9-11(5-6-12(13)3-1)10-14-15-7-8-16-14/h1-6,9H,7-8,10H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50222218
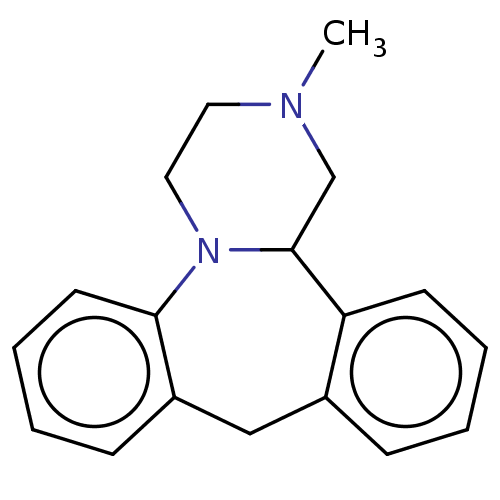
(CHEBI:51137 | Mianserin)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226927

(CHEMBL544701)Show InChI InChI=1S/C13H14N2/c1-2-4-12-8-10(7-11(12)3-1)9-13-14-5-6-15-13/h1-4,7H,5-6,8-9H2,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50454831

(Napamezole)Show InChI InChI=1S/C14H16N2/c1-2-4-13-9-11(5-6-12(13)3-1)10-14-15-7-8-16-14/h1-4,9H,5-8,10H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]prazosin from alpha-1 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226930
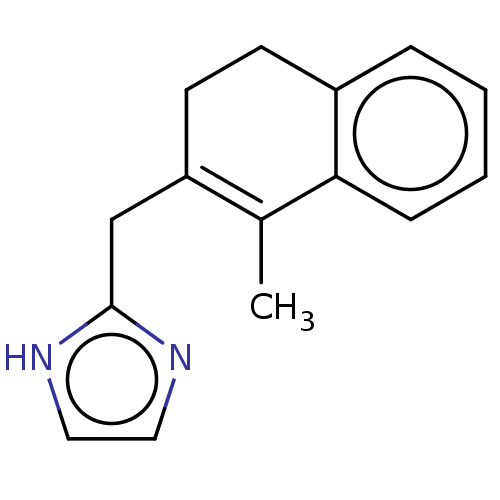
(CHEMBL35345)Show InChI InChI=1S/C15H16N2/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12/h2-5,8-9H,6-7,10H2,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226926
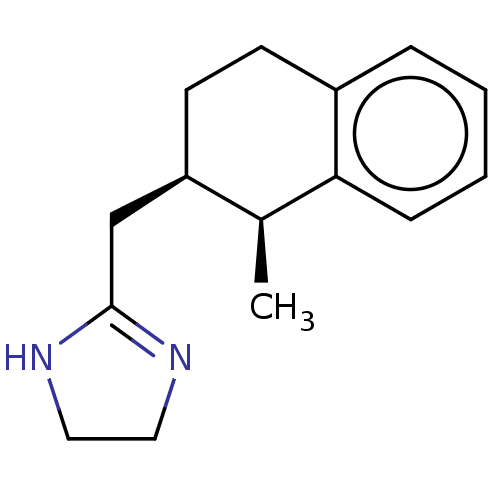
(CHEMBL544706)Show SMILES Cl.[H][C@]1(CC2=NCCN2)CCc2ccccc2[C@H]1C |r,t:3| Show InChI InChI=1S/C15H20N2.ClH/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12;/h2-5,11,13H,6-10H2,1H3,(H,16,17);1H/t11-,13+;/m0./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50226929

(CHEMBL545646)Show InChI InChI=1S/C15H18N2/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12/h2-5H,6-10H2,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]prazosin from alpha-1 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]prazosin from alpha-1 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226921

(CHEMBL544704)Show InChI InChI=1S/C16H20N2/c1-12-14(11-16-17-9-4-10-18-16)8-7-13-5-2-3-6-15(12)13/h2-3,5-6H,4,7-11H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226923

(CHEMBL544705)Show SMILES Cl.[H][C@]1(CC2=NCCN2)CCc2ccccc2[C@@H]1C |r,t:3| Show InChI InChI=1S/C15H20N2.ClH/c1-11-13(10-15-16-8-9-17-15)7-6-12-4-2-3-5-14(11)12;/h2-5,11,13H,6-10H2,1H3,(H,16,17);1H/t11-,13-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226925

(CHEMBL553103)Show InChI InChI=1S/C16H20N2/c1-12-14(11-16-17-9-10-18(16)2)8-7-13-5-3-4-6-15(12)13/h3-6H,7-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226931

(CHEMBL553368)Show InChI InChI=1S/C17H22N2/c1-3-19-11-10-18-17(19)12-15-9-8-14-6-4-5-7-16(14)13(15)2/h4-7H,3,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021345
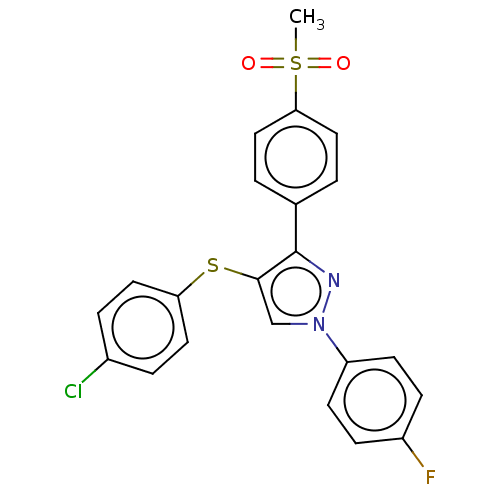
(CHEMBL3287928)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H16ClFN2O2S2/c1-30(27,28)20-12-2-15(3-13-20)22-21(29-19-10-4-16(23)5-11-19)14-26(25-22)18-8-6-17(24)7-9-18/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021331
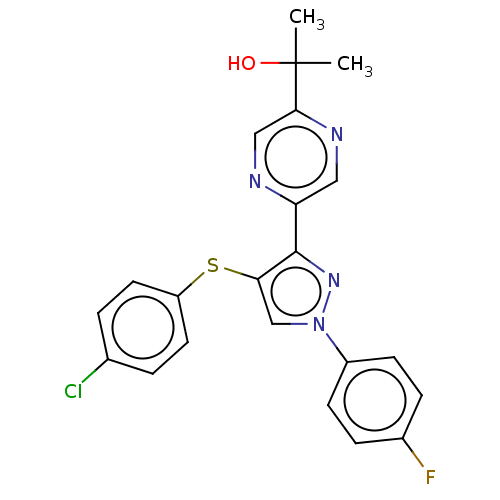
(CHEMBL3287930)Show SMILES CC(C)(O)c1cnc(cn1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H18ClFN4OS/c1-22(2,29)20-12-25-18(11-26-20)21-19(30-17-9-3-14(23)4-10-17)13-28(27-21)16-7-5-15(24)6-8-16/h3-13,29H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50226922

(CHEMBL543535)Show SMILES Cl.CC1CN=C(CC2=C(C)c3ccccc3CC2)N1 |c:6,t:3| Show InChI InChI=1S/C16H20N2.ClH/c1-11-10-17-16(18-11)9-14-8-7-13-5-3-4-6-15(13)12(14)2;/h3-6,11H,7-10H2,1-2H3,(H,17,18);1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]clonidine from alpha-2 adrenergic receptor in rat brain homogenates in vitro |
J Med Chem 30: 1482-9 (1987)
BindingDB Entry DOI: 10.7270/Q2WS8WGP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350538
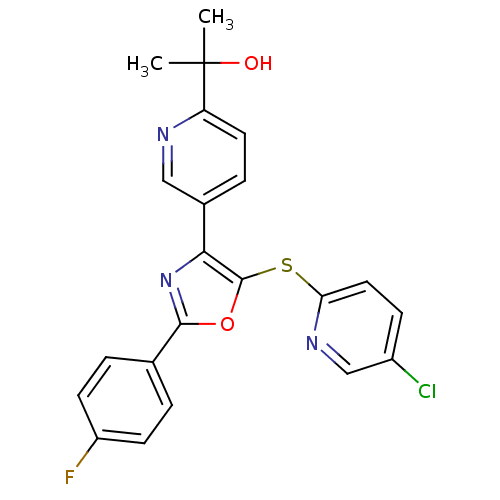
(CHEMBL1812717)Show SMILES CC(C)(O)c1ccc(cn1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17ClFN3O2S/c1-22(2,28)17-9-5-14(11-25-17)19-21(30-18-10-6-15(23)12-26-18)29-20(27-19)13-3-7-16(24)8-4-13/h3-12,28H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021346
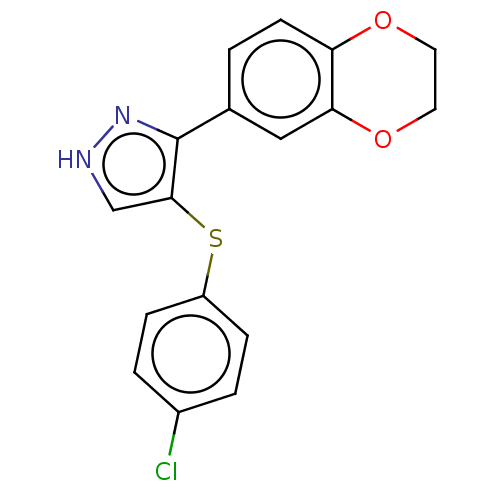
(CHEMBL3287926)Show InChI InChI=1S/C17H13ClN2O2S/c18-12-2-4-13(5-3-12)23-16-10-19-20-17(16)11-1-6-14-15(9-11)22-8-7-21-14/h1-6,9-10H,7-8H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021329
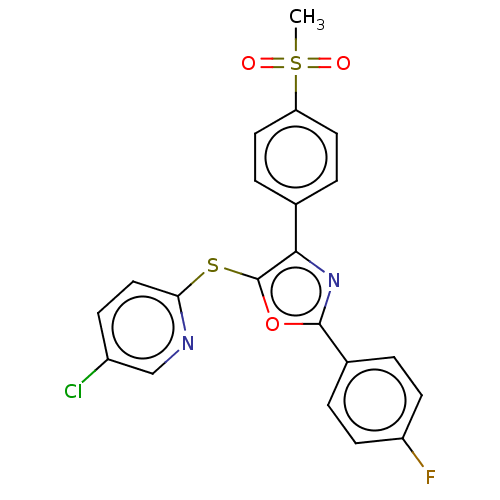
(CHEMBL3287932)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H14ClFN2O3S2/c1-30(26,27)17-9-4-13(5-10-17)19-21(29-18-11-6-15(22)12-24-18)28-20(25-19)14-2-7-16(23)8-3-14/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021344
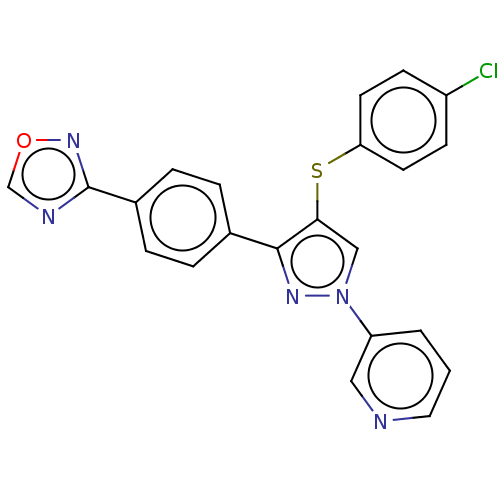
(CHEMBL3287929)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc(cc2)-c2ncon2)-c2cccnc2)cc1 Show InChI InChI=1S/C22H14ClN5OS/c23-17-7-9-19(10-8-17)30-20-13-28(18-2-1-11-24-12-18)26-21(20)15-3-5-16(6-4-15)22-25-14-29-27-22/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021334
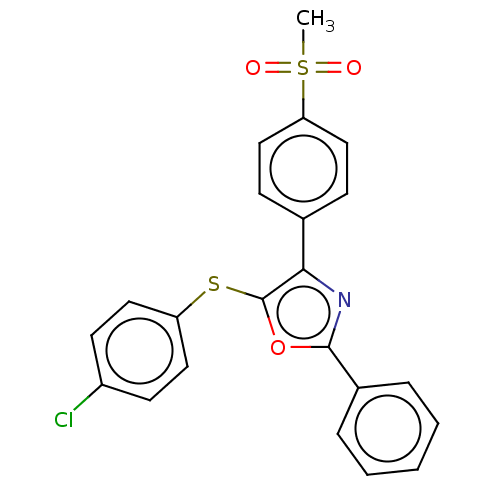
(CHEMBL3287931)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C22H16ClNO3S2/c1-29(25,26)19-13-7-15(8-14-19)20-22(28-18-11-9-17(23)10-12-18)27-21(24-20)16-5-3-2-4-6-16/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021330
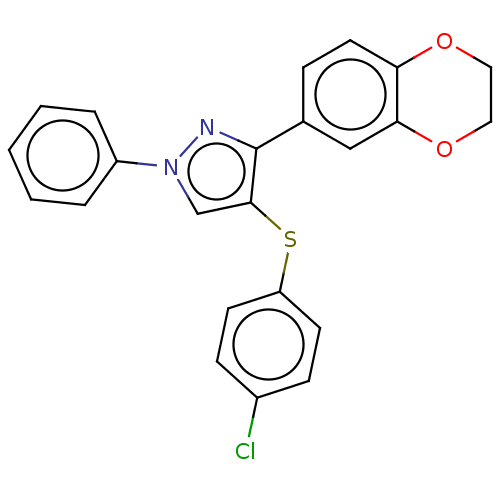
(CHEMBL3287927)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc3OCCOc3c2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H17ClN2O2S/c24-17-7-9-19(10-8-17)29-22-15-26(18-4-2-1-3-5-18)25-23(22)16-6-11-20-21(14-16)28-13-12-27-20/h1-11,14-15H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Monoacylglycerol lipase ABHD6
(Mus musculus (mouse)) | BDBM26736
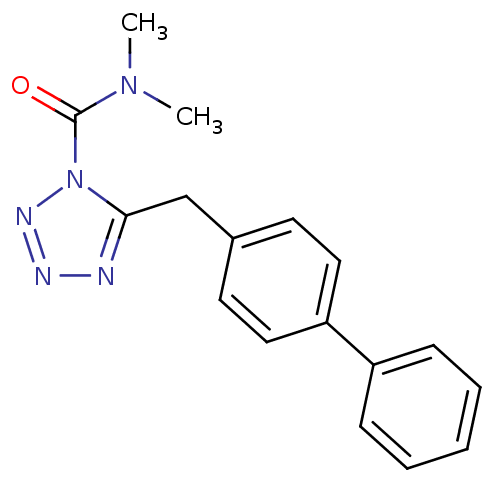
(CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phen...)Show InChI InChI=1S/C17H17N5O/c1-21(2)17(23)22-16(18-19-20-22)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11H,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 22 |
The Scripps Research Institute
| Assay Description
Serine hydrolase targets were recombinantly expressed in COS-7 cells by transient transfection. IC50 values were obtained by competitive ABPP with F... |
J Am Chem Soc 128: 9699-704 (2006)
Article DOI: 10.1021/ja062999h
BindingDB Entry DOI: 10.7270/Q20K26VX |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434318

(CHEMBL2386566)Show SMILES CN(C)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H22ClN3OS/c1-25(2)21(27)19-12-18(19)14-4-6-15(7-5-14)20-22(26(3)13-24-20)28-17-10-8-16(23)9-11-17/h4-11,13,18-19H,12H2,1-3H3/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434329

(CHEMBL2386554)Show SMILES Cn1cc(Sc2ccc(Cl)cc2)c(n1)-c1ccc2ccc(nc2c1)C(N)=O Show InChI InChI=1S/C20H15ClN4OS/c1-25-11-18(27-15-7-5-14(21)6-8-15)19(24-25)13-3-2-12-4-9-16(20(22)26)23-17(12)10-13/h2-11H,1H3,(H2,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434316
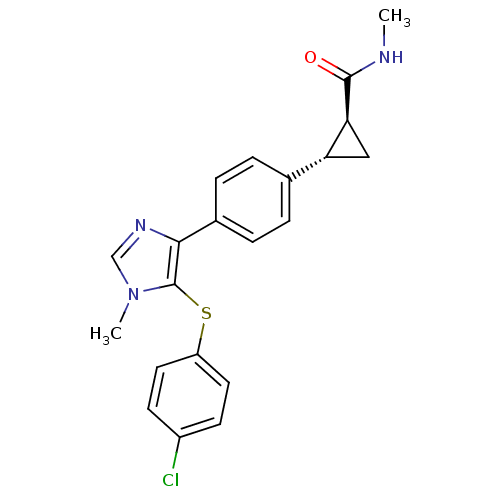
(CHEMBL2386568)Show SMILES CNC(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H20ClN3OS/c1-23-20(26)18-11-17(18)13-3-5-14(6-4-13)19-21(25(2)12-24-19)27-16-9-7-15(22)8-10-16/h3-10,12,17-18H,11H2,1-2H3,(H,23,26)/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434329

(CHEMBL2386554)Show SMILES Cn1cc(Sc2ccc(Cl)cc2)c(n1)-c1ccc2ccc(nc2c1)C(N)=O Show InChI InChI=1S/C20H15ClN4OS/c1-25-11-18(27-15-7-5-14(21)6-8-15)19(24-25)13-3-2-12-4-9-16(20(22)26)23-17(12)10-13/h2-11H,1H3,(H2,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434318

(CHEMBL2386566)Show SMILES CN(C)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H22ClN3OS/c1-25(2)21(27)19-12-18(19)14-4-6-15(7-5-14)20-22(26(3)13-24-20)28-17-10-8-16(23)9-11-17/h4-11,13,18-19H,12H2,1-3H3/t18-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434317

(CHEMBL2386567)Show SMILES Cn1cnc(c1Sc1ccc(Cl)cc1)-c1ccc(cc1)[C@H]1C[C@@H]1C(N)=O |r| Show InChI InChI=1S/C20H18ClN3OS/c1-24-11-23-18(20(24)26-15-8-6-14(21)7-9-15)13-4-2-12(3-5-13)16-10-17(16)19(22)25/h2-9,11,16-17H,10H2,1H3,(H2,22,25)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434315
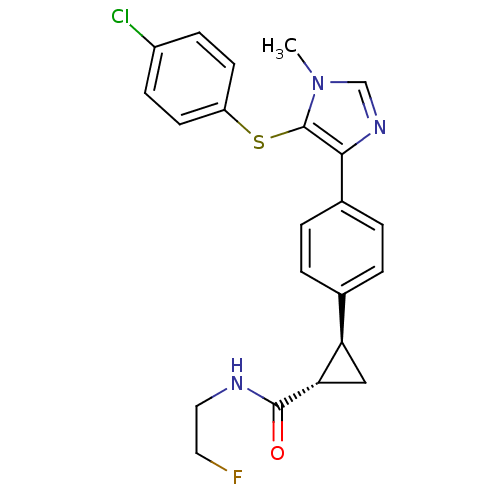
(CHEMBL2386569)Show SMILES Cn1cnc(c1Sc1ccc(Cl)cc1)-c1ccc(cc1)[C@H]1C[C@@H]1C(=O)NCCF |r| Show InChI InChI=1S/C22H21ClFN3OS/c1-27-13-26-20(22(27)29-17-8-6-16(23)7-9-17)15-4-2-14(3-5-15)18-12-19(18)21(28)25-11-10-24/h2-9,13,18-19H,10-12H2,1H3,(H,25,28)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434313
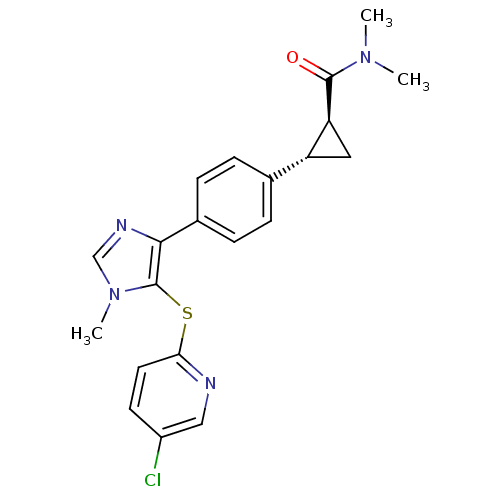
(CHEMBL2386571)Show SMILES CN(C)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C21H21ClN4OS/c1-25(2)20(27)17-10-16(17)13-4-6-14(7-5-13)19-21(26(3)12-24-19)28-18-9-8-15(22)11-23-18/h4-9,11-12,16-17H,10H2,1-3H3/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434322
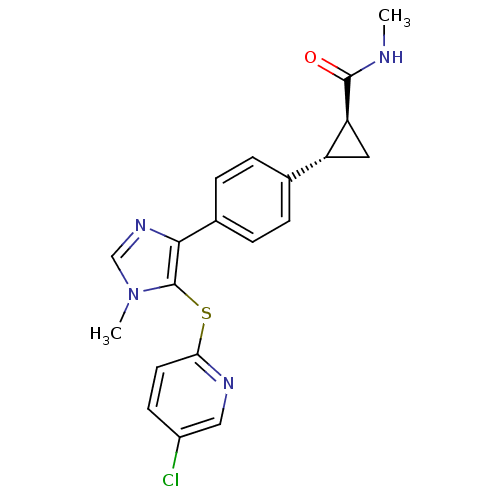
(CHEMBL2386562)Show SMILES CNC(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C20H19ClN4OS/c1-22-19(26)16-9-15(16)12-3-5-13(6-4-12)18-20(25(2)11-24-18)27-17-8-7-14(21)10-23-17/h3-8,10-11,15-16H,9H2,1-2H3,(H,22,26)/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434314
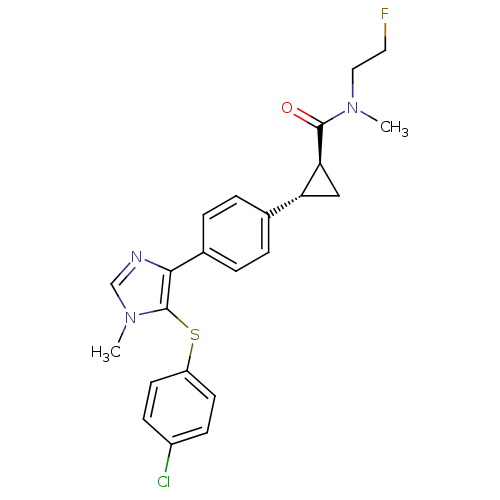
(CHEMBL2386570)Show SMILES CN(CCF)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H23ClFN3OS/c1-27(12-11-25)22(29)20-13-19(20)15-3-5-16(6-4-15)21-23(28(2)14-26-21)30-18-9-7-17(24)8-10-18/h3-10,14,19-20H,11-13H2,1-2H3/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434314

(CHEMBL2386570)Show SMILES CN(CCF)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H23ClFN3OS/c1-27(12-11-25)22(29)20-13-19(20)15-3-5-16(6-4-15)21-23(28(2)14-26-21)30-18-9-7-17(24)8-10-18/h3-10,14,19-20H,11-13H2,1-2H3/t19-,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50032599
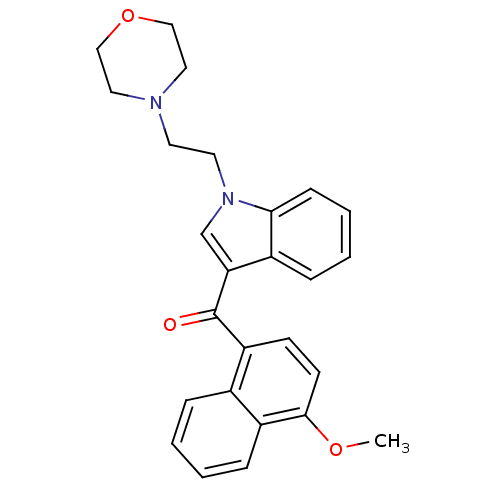
((4-Methoxy-naphthalen-1-yl)-[1-(2-morpholin-4-yl-e...)Show SMILES COc1ccc(C(=O)c2cn(CCN3CCOCC3)c3ccccc23)c2ccccc12 Show InChI InChI=1S/C26H26N2O3/c1-30-25-11-10-22(19-6-2-3-8-21(19)25)26(29)23-18-28(24-9-5-4-7-20(23)24)13-12-27-14-16-31-17-15-27/h2-11,18H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division
Curated by ChEMBL
| Assay Description
Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. |
J Med Chem 38: 3094-105 (1995)
BindingDB Entry DOI: 10.7270/Q2DN442Z |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434315

(CHEMBL2386569)Show SMILES Cn1cnc(c1Sc1ccc(Cl)cc1)-c1ccc(cc1)[C@H]1C[C@@H]1C(=O)NCCF |r| Show InChI InChI=1S/C22H21ClFN3OS/c1-27-13-26-20(22(27)29-17-8-6-16(23)7-9-17)15-4-2-14(3-5-15)18-12-19(18)21(28)25-11-10-24/h2-9,13,18-19H,10-12H2,1H3,(H,25,28)/t18-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434316

(CHEMBL2386568)Show SMILES CNC(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H20ClN3OS/c1-23-20(26)18-11-17(18)13-3-5-14(6-4-13)19-21(25(2)12-24-19)27-16-9-7-15(22)8-10-16/h3-10,12,17-18H,11H2,1-2H3,(H,23,26)/t17-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434320
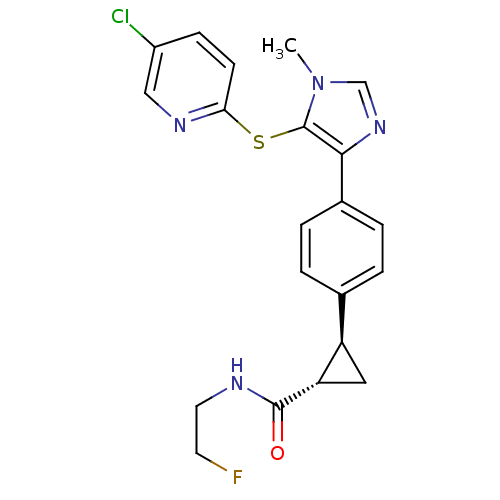
(CHEMBL2386564)Show SMILES Cn1cnc(c1Sc1ccc(Cl)cn1)-c1ccc(cc1)[C@H]1C[C@@H]1C(=O)NCCF |r| Show InChI InChI=1S/C21H20ClFN4OS/c1-27-12-26-19(21(27)29-18-7-6-15(22)11-25-18)14-4-2-13(3-5-14)16-10-17(16)20(28)24-9-8-23/h2-7,11-12,16-17H,8-10H2,1H3,(H,24,28)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434317

(CHEMBL2386567)Show SMILES Cn1cnc(c1Sc1ccc(Cl)cc1)-c1ccc(cc1)[C@H]1C[C@@H]1C(N)=O |r| Show InChI InChI=1S/C20H18ClN3OS/c1-24-11-23-18(20(24)26-15-8-6-14(21)7-9-15)13-4-2-12(3-5-13)16-10-17(16)19(22)25/h2-9,11,16-17H,10H2,1H3,(H2,22,25)/t16-,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50434313

(CHEMBL2386571)Show SMILES CN(C)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C21H21ClN4OS/c1-25(2)20(27)17-10-16(17)13-4-6-14(7-5-13)19-21(26(3)12-24-19)28-18-9-8-15(22)11-23-18/h4-9,11-12,16-17H,10H2,1-3H3/t16-,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50503340
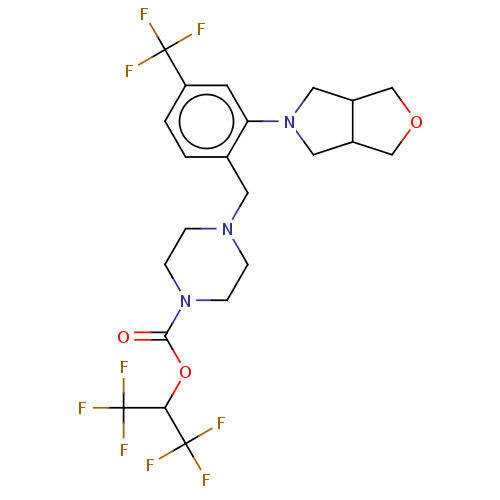
(CHEMBL4468636)Show SMILES FC(F)(F)C(OC(=O)N1CCN(Cc2ccc(cc2N2CC3COCC3C2)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C22H24F9N3O3/c23-20(24,25)16-2-1-13(17(7-16)34-9-14-11-36-12-15(14)10-34)8-32-3-5-33(6-4-32)19(35)37-18(21(26,27)28)22(29,30)31/h1-2,7,14-15,18H,3-6,8-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abide Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of MGLL derived from human PC3 cell lysates preincubated for 30 mins followed by JW912 addition after 30 mins by gel-based ABPP assay |
J Med Chem 61: 9062-9084 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00951
BindingDB Entry DOI: 10.7270/Q2TH8QZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data