

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50162625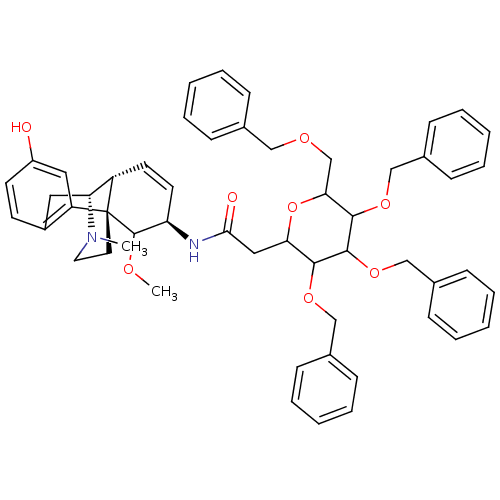 (Benzyl derivative of M6G | CHEMBL366917) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to recombinant human Opioid receptor mu 1 | Bioorg Med Chem Lett 15: 1583-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.072 BindingDB Entry DOI: 10.7270/Q2HT2Q2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50162622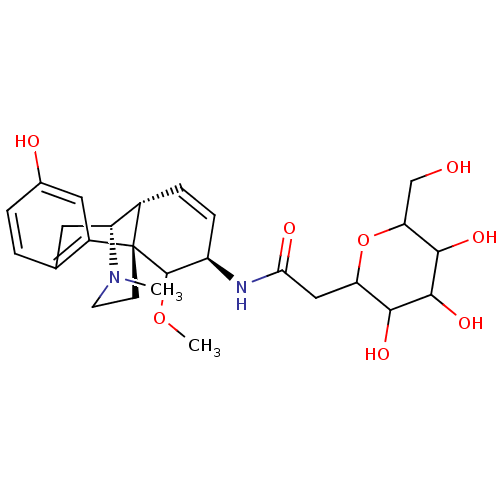 (CHEMBL362202 | Deprotected cogener of M6G) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to recombinant human Opioid receptor mu 1 | Bioorg Med Chem Lett 15: 1583-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.072 BindingDB Entry DOI: 10.7270/Q2HT2Q2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50162624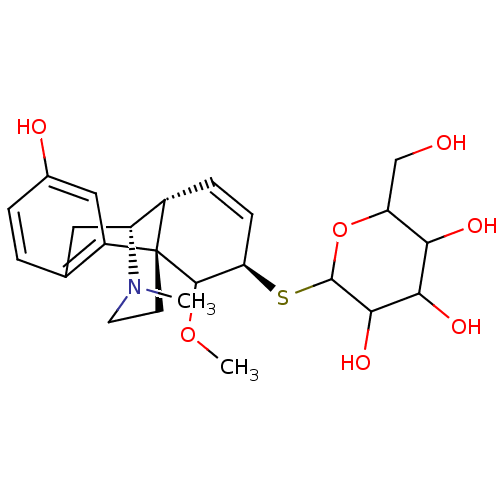 (CHEMBL359644 | M6G thiosaccharide analogue) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to recombinant human Opioid receptor mu 1 | Bioorg Med Chem Lett 15: 1583-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.072 BindingDB Entry DOI: 10.7270/Q2HT2Q2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50162626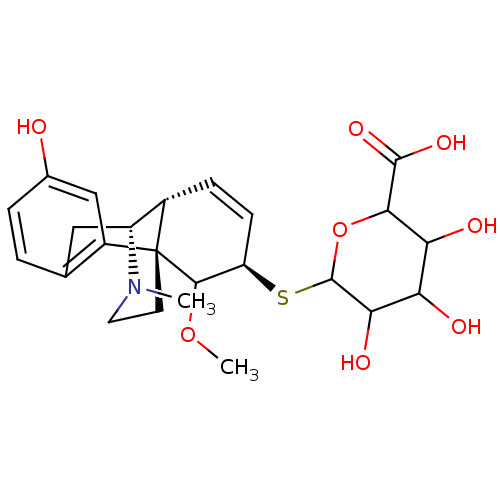 (CHEMBL359534 | M6G thiosaccharide analogue) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to recombinant human Opioid receptor mu 1 | Bioorg Med Chem Lett 15: 1583-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.072 BindingDB Entry DOI: 10.7270/Q2HT2Q2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50370478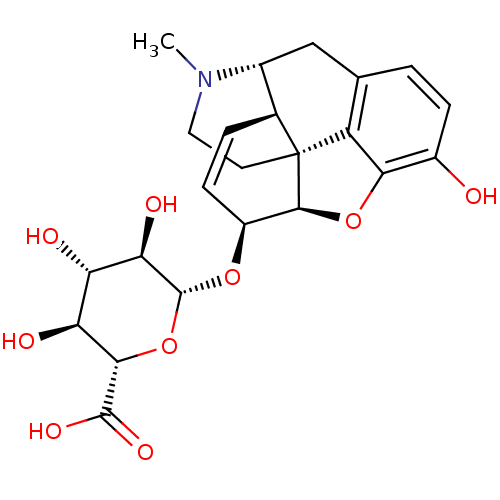 (MORPHINE-6-GLUCURONIDE | Morphine 6-Glucuronide(Mi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to recombinant human Opioid receptor mu 1 | Bioorg Med Chem Lett 15: 1583-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.072 BindingDB Entry DOI: 10.7270/Q2HT2Q2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493380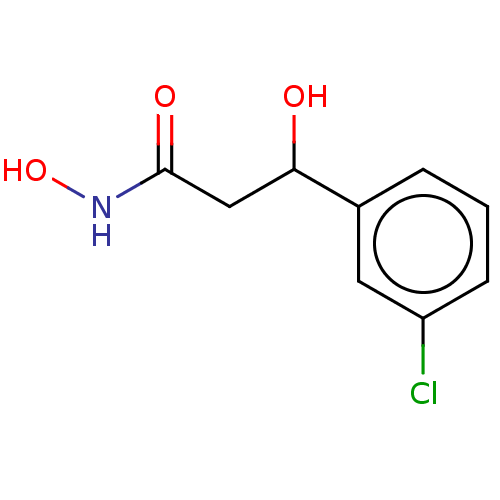 (CHEMBL2425469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493373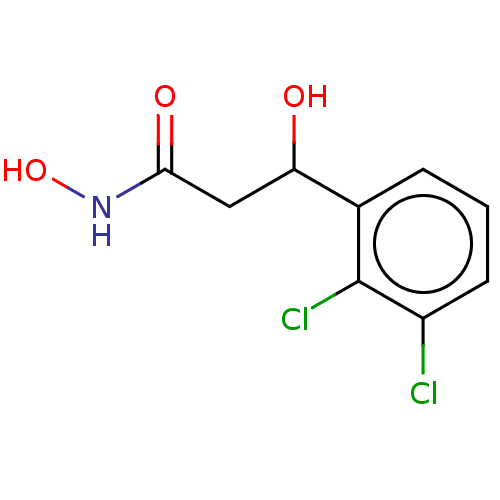 (CHEMBL2425478) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493380 (CHEMBL2425469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264501 (CHEMBL253715 | CHEMBL484670 | N-(5-N-Methyl-10H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264595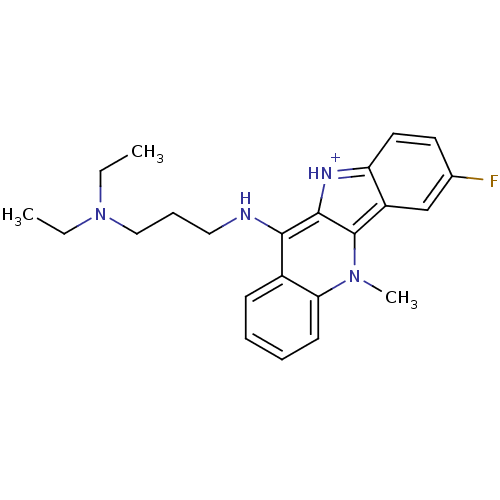 (CHEMBL491633 | N-(7-Fluoro-5-N-methyl-10H-indolo[3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264499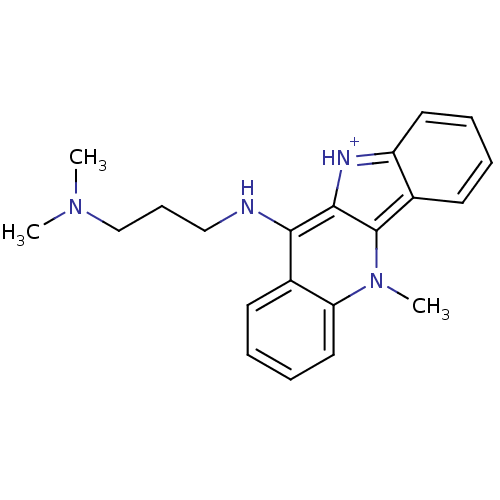 (CHEMBL253508 | CHEMBL507633 | N-(5-N-Methyl-10H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493373 (CHEMBL2425478) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264635 (CHEMBL490013 | N-(7,9-Difluoro-5-N-methyl-10H-indo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264593 (11-(3-(dimethylamino)propylamino)-7-fluoro-5-methy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493380 (CHEMBL2425469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 intact cell assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264535 (CHEMBL482414 | N-(5-N-Methyl-benzofuro[3,2-b]quino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493365 (CHEMBL2425479) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50264633 (CHEMBL489811 | N-(7,9-Difluoro-5-N-methyl-10H-indo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50176906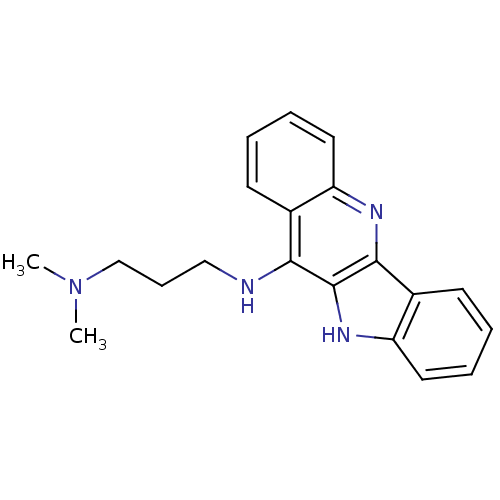 (CHEMBL219728 | N'-(10H-indolo[3,2-b]quinolin-11-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of telomerase in human MCF7 cells by cell-free telomerase repeat amplification protocol assay | J Med Chem 51: 6381-92 (2008) Article DOI: 10.1021/jm800497p BindingDB Entry DOI: 10.7270/Q2319VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075474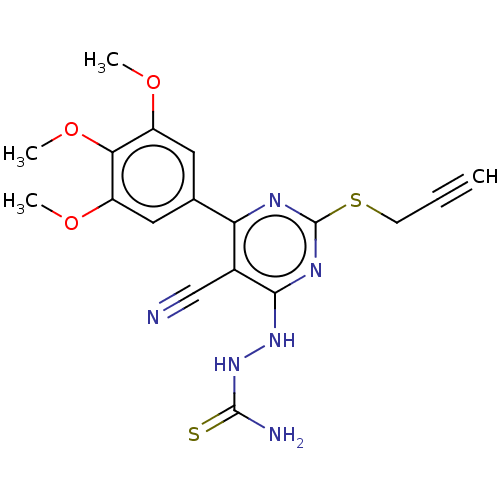 (CHEMBL3415352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493373 (CHEMBL2425478) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 intact cell assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493374 (CHEMBL2425482) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075573 (CHEMBL3415354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075574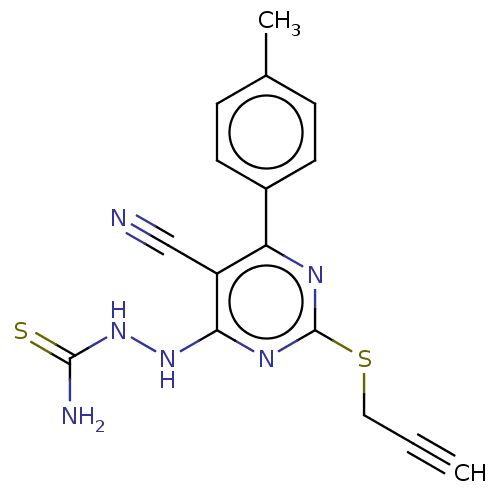 (CHEMBL3415353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxyanthranilate 3,4-dioxygenase (Homo sapiens (Human)) | BDBM50446510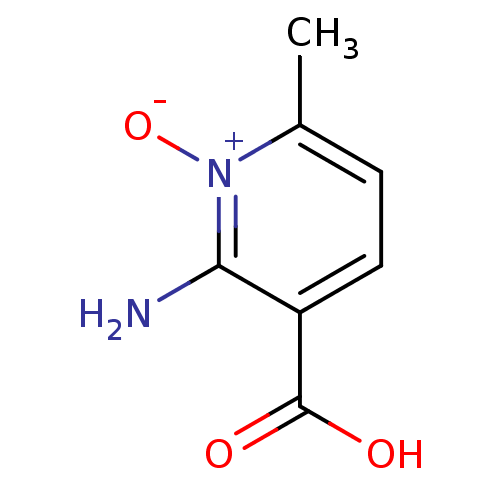 (CHEMBL3110069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Inhibition of 3-HAO in human brain homogenates using [1-14C]-3-HANA as substrate assessed as [14C]-QUIN production after 1 hr by liquid scintillation... | J Med Chem 56: 9482-95 (2014) Article DOI: 10.1021/jm401249c BindingDB Entry DOI: 10.7270/Q21N82MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497256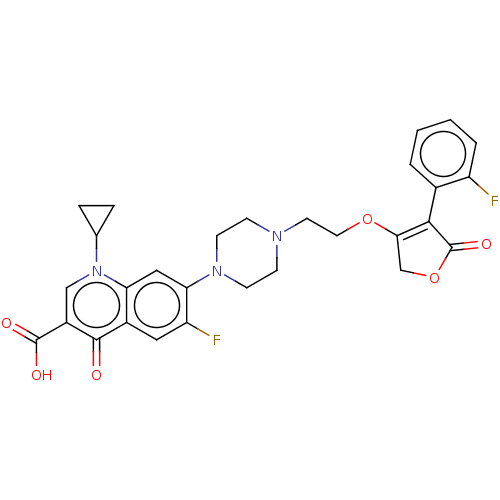 (CHEMBL3298511) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase assessed as reduction in enzyme-mediated DNA supercoiling using relaxed pBR322 substrate incubated for 60 m... | Bioorg Med Chem 22: 3620-8 (2014) Article DOI: 10.1016/j.bmc.2014.05.018 BindingDB Entry DOI: 10.7270/Q2NV9N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493375 (CHEMBL2425465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493381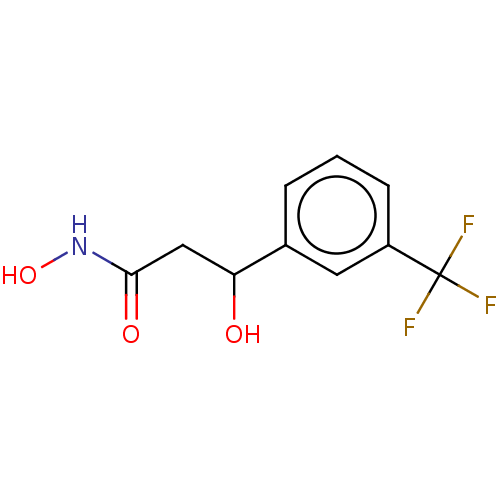 (CHEMBL2425471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364 (CHEMBL2425480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493365 (CHEMBL2425479) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 intact cell assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075575 (CHEMBL3415351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075569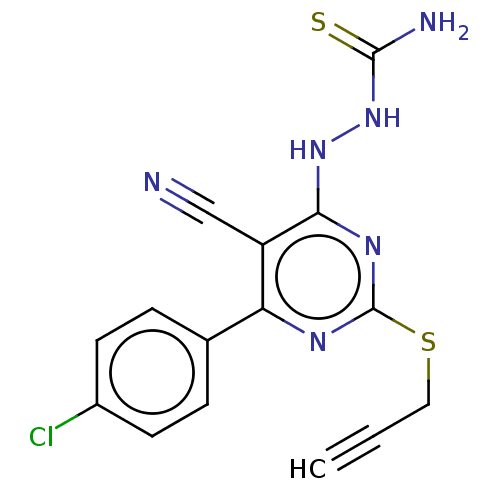 (CHEMBL3415357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075567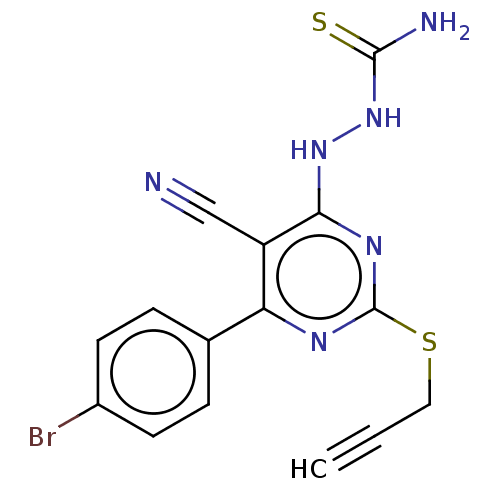 (CHEMBL3415359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075568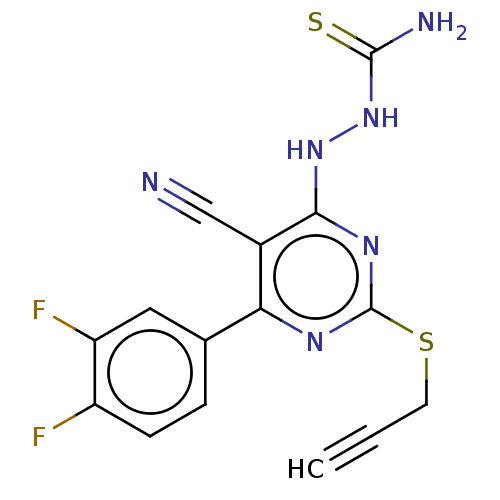 (CHEMBL3415358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075571 (CHEMBL3415355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042195 (CHEMBL3360188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075570 (CHEMBL3415356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042274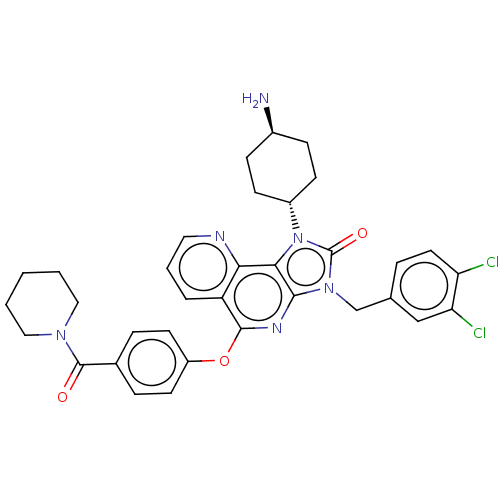 (CHEMBL3360682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075564 (CHEMBL3415361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075562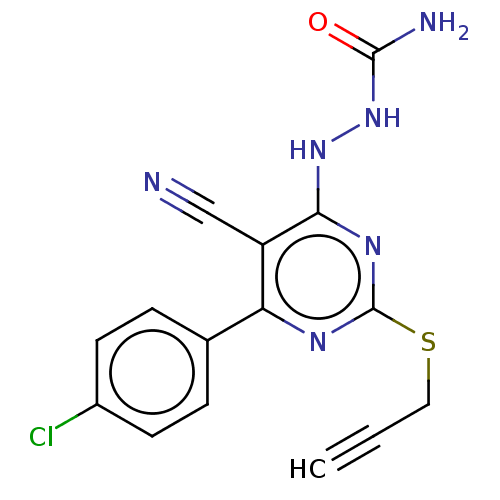 (CHEMBL3415362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxyanthranilate 3,4-dioxygenase (Rattus norvegicus) | BDBM50446510 (CHEMBL3110069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Inhibition of 3-HAO in Sprague-Dawley rat brain homogenates using [1-14C]-3-HANA as substrate assessed as [14C]-QUIN production after 1 hr by liquid ... | J Med Chem 56: 9482-95 (2014) Article DOI: 10.1021/jm401249c BindingDB Entry DOI: 10.7270/Q21N82MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497254 (CHEMBL3298605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase assessed as reduction in enzyme-mediated DNA supercoiling using relaxed pBR322 substrate incubated for 60 m... | Bioorg Med Chem 22: 3620-8 (2014) Article DOI: 10.1016/j.bmc.2014.05.018 BindingDB Entry DOI: 10.7270/Q2NV9N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042189 (CHEMBL3360676) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075487 (CHEMBL3415540) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075560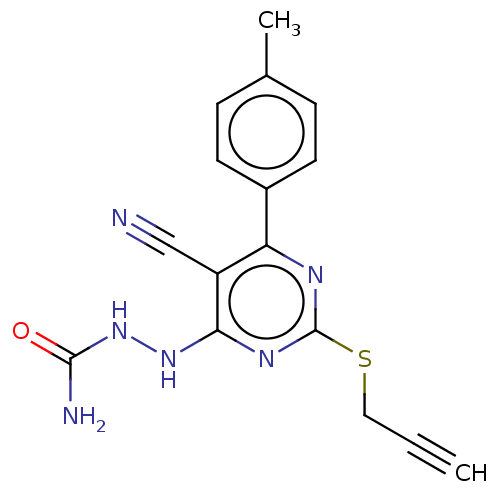 (CHEMBL3415364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493384 (CHEMBL2425474) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease extracted from Helicobacter pylori ATCC 43504 assessed as ammonia production preincubated for 1.5 hrs by indophenol-based method | Eur J Med Chem 68: 212-21 (2013) Article DOI: 10.1016/j.ejmech.2013.07.047 BindingDB Entry DOI: 10.7270/Q28G8PNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50592734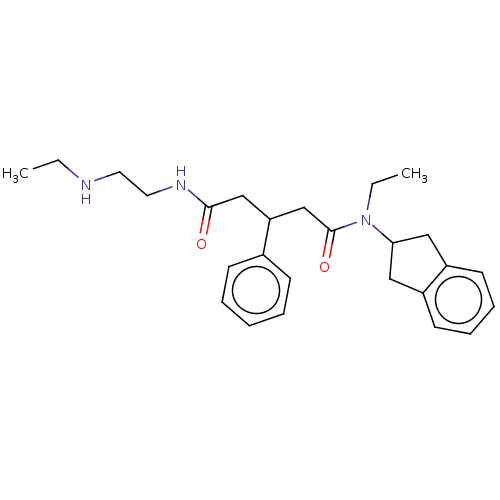 (CHEMBL5176164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128880 BindingDB Entry DOI: 10.7270/Q2ST7TVT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50592731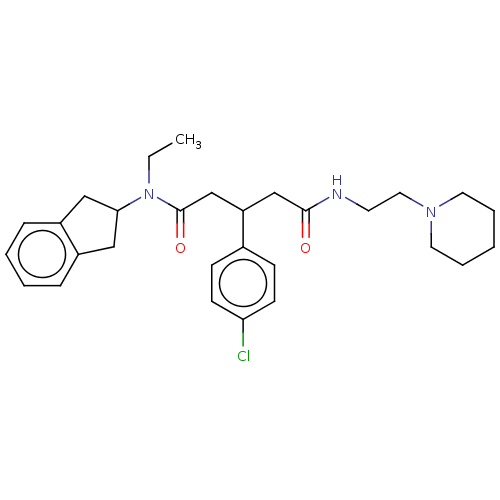 (CHEMBL5202341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128880 BindingDB Entry DOI: 10.7270/Q2ST7TVT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50075565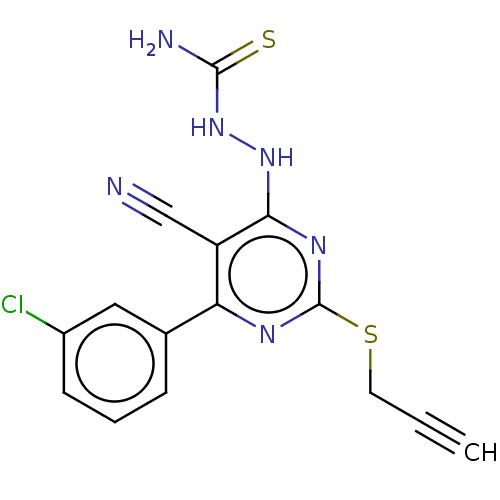 (CHEMBL3415360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of recombinant LSD1 (157 to 852) (unknown origin) transfected in Escherichia coli BL21(DE) by fluorescence assay | J Med Chem 58: 1705-16 (2015) Article DOI: 10.1021/acs.jmedchem.5b00037 BindingDB Entry DOI: 10.7270/Q2CC12CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042192 (CHEMBL3360673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |