

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069019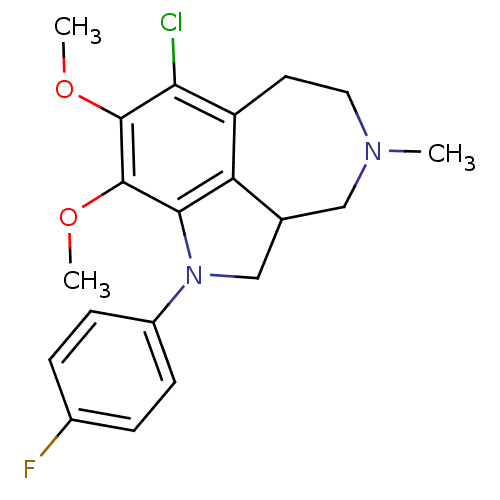 (7-Chloro-1-(4-fluoro-phenyl)-8,9-dimethoxy-4-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M4 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M2 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 5049-52 (2007) Article DOI: 10.1021/jm070231h BindingDB Entry DOI: 10.7270/Q29Z935Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The binding affinity was measured on 5-hydroxytryptamine 2 receptor in rat brain tissue | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Dopamine receptor D2 | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M5 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The compound was tested for its binding affinity towards 5-hydroxytryptamine 2 receptor by displacing [3H]ketanserin radioligand in rat cerebral cort... | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18605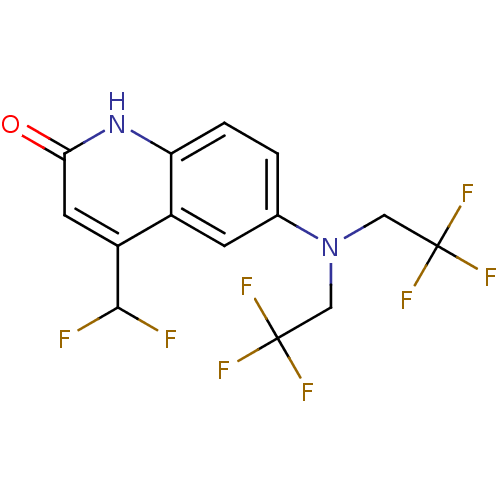 (6-[bis(2,2,2-trifluoroethyl)amino]-4-(difluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069011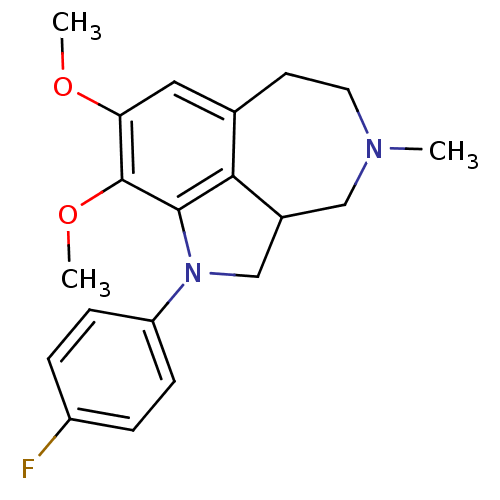 (1-(4-Fluoro-phenyl)-8,9-dimethoxy-4-methyl-2,2a,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001876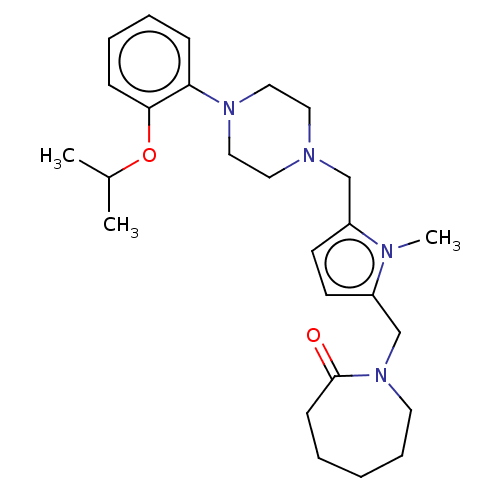 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82403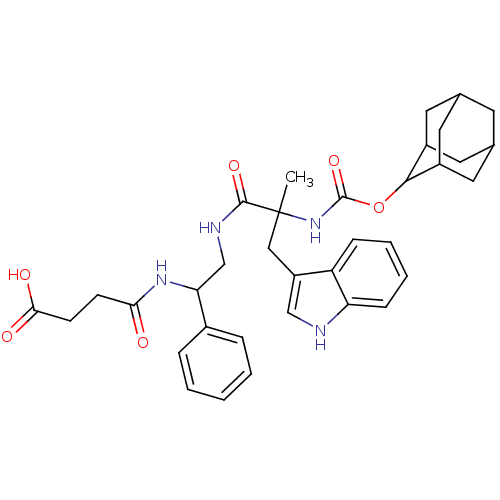 (CAS_108186 | CI-988 | NSC_108186) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82404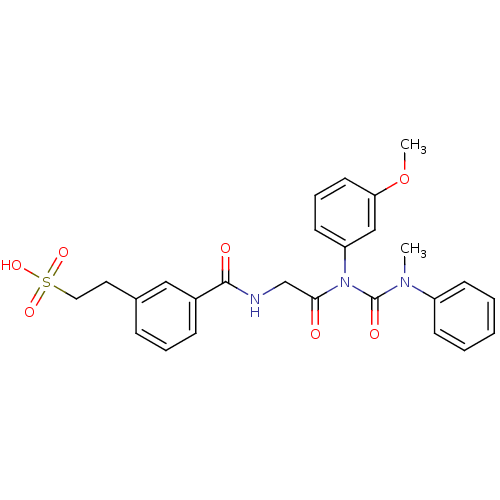 (Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18607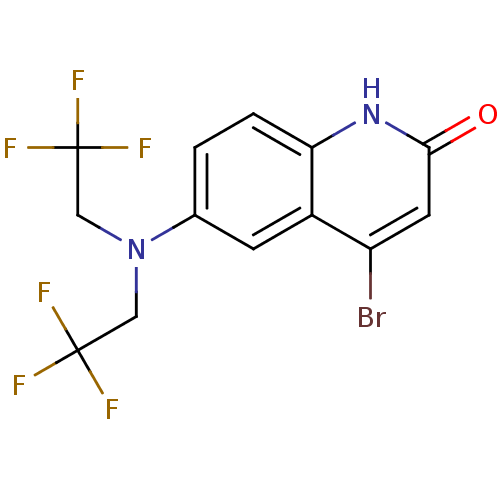 (6-[bis(2,2,2-trifluoroethyl)amino]-4-bromo-1,2-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214923 ((Z)-5-(4-fluoro-2-(hydroxymethyl)benzylidene)-10-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18578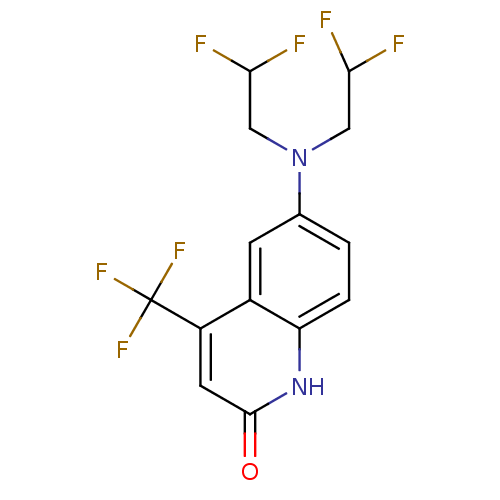 (6-[bis(2,2-difluoroethyl)amino]-4-(trifluoromethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -55.2 | n/a | n/a | 0.400 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18606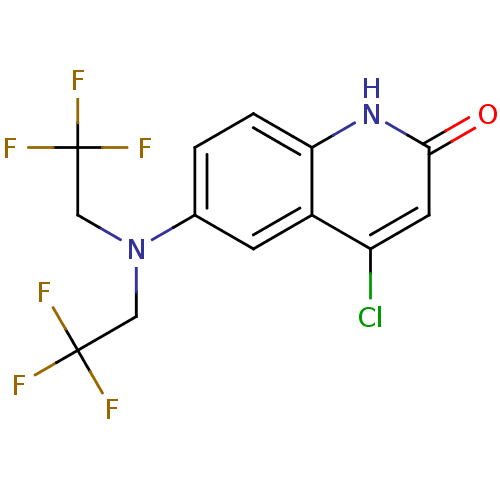 (6-[bis(2,2,2-trifluoroethyl)amino]-4-chloro-1,2-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1527-31 (2007) Article DOI: 10.1016/j.bmcl.2007.01.001 BindingDB Entry DOI: 10.7270/Q2XS5SP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214929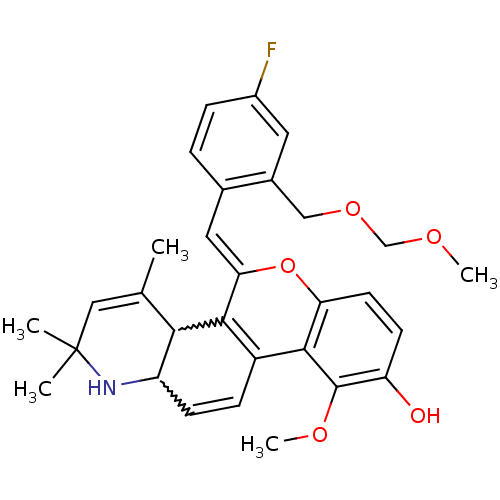 ((Z)-5-(4-fluoro-2-((methoxymethoxy)methyl)benzylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069016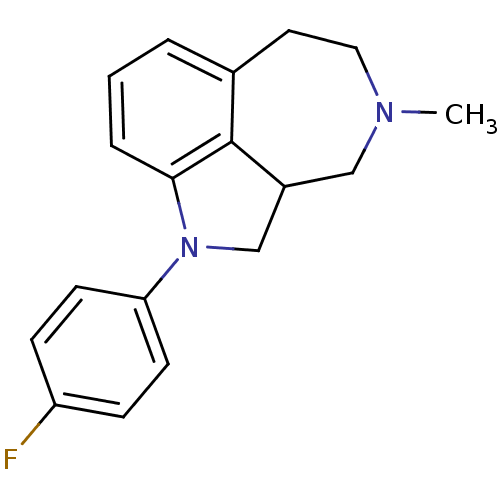 (1-(4-Fluoro-phenyl)-4-methyl-2,2a,3,4,5,6-hexahydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001855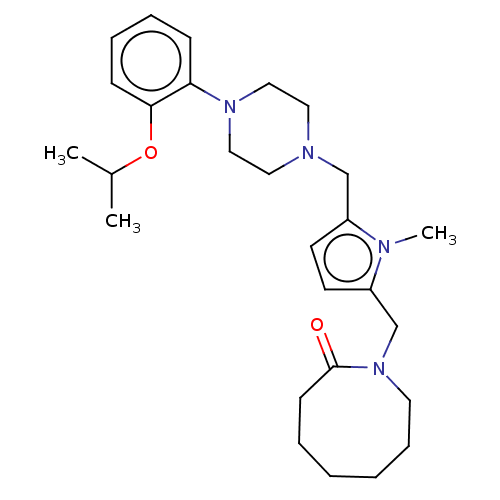 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415153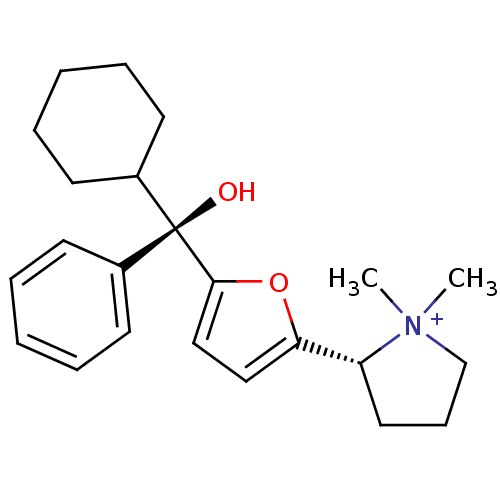 (CHEMBL569307) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity determined in radioreceptor binding assay by using [3H]ketanserin radioligand against 5-hydroxytryptamine 2 receptor | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869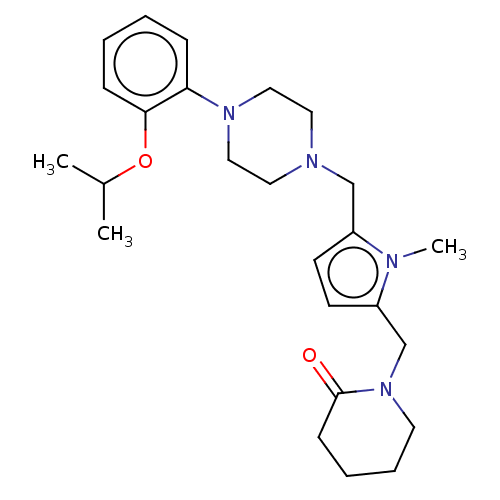 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214949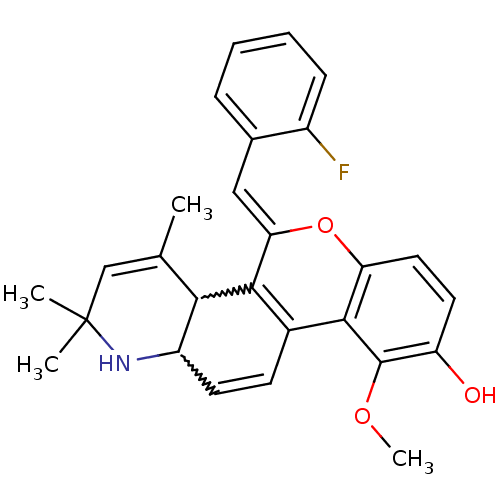 ((Z)-5-(2-fluorobenzylidene)-10-methoxy-2,2,4-trime...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82404 (Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM419188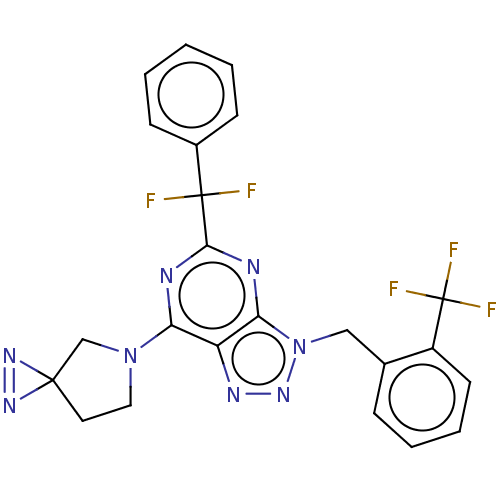 (5-[Difluoro(phenyl)methyl]-7-(1,2,5-triazaspiro[2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElm... | US Patent US10457684 (2019) BindingDB Entry DOI: 10.7270/Q2HM5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214919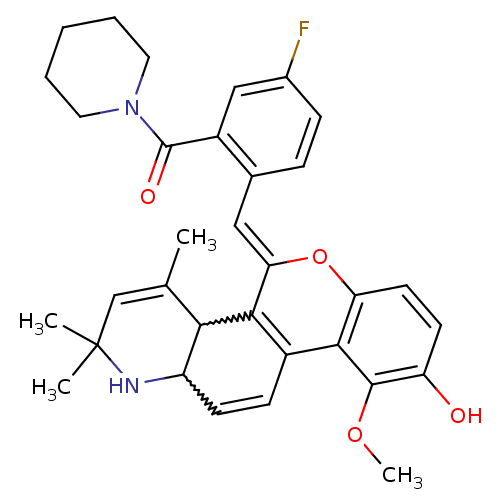 ((Z)-(5-fluoro-2-((9-hydroxy-10-methoxy-2,2,4-trime...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214932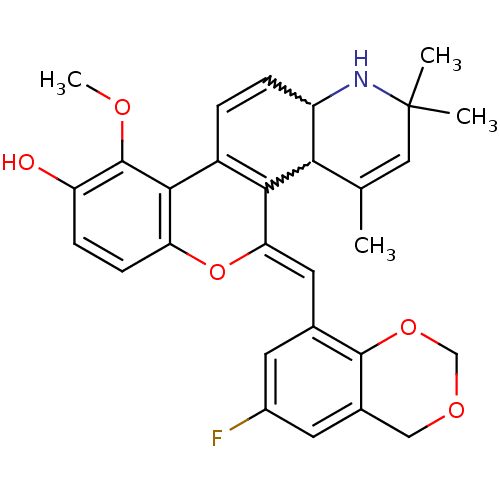 ((Z)-5-((6-fluoro-4H-benzo[d][1,3]dioxin-8-yl)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18216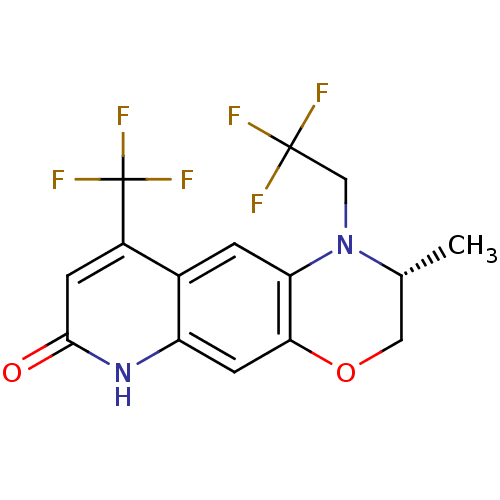 ((2R)-2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 18: 2967-71 (2008) Article DOI: 10.1016/j.bmcl.2008.03.062 BindingDB Entry DOI: 10.7270/Q2R2128X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 18: 2967-71 (2008) Article DOI: 10.1016/j.bmcl.2008.03.062 BindingDB Entry DOI: 10.7270/Q2R2128X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM419309 (2-[[5-tert-Butyl-7-(3,3-difluoropyrrolidin-1-yl)tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description he affinity of the compounds of the invention for cannabinoid receptors was determined using recommended amounts of membrane preparations (PerkinElme... | US Patent US10457685 (2019) BindingDB Entry DOI: 10.7270/Q2CV4M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069012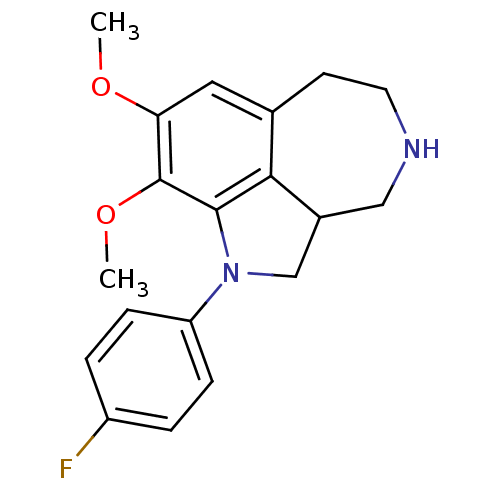 (1-(4-Fluoro-phenyl)-8,9-dimethoxy-2,2a,3,4,5,6-hex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50377413 (CHEMBL257379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 18: 2967-71 (2008) Article DOI: 10.1016/j.bmcl.2008.03.062 BindingDB Entry DOI: 10.7270/Q2R2128X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001889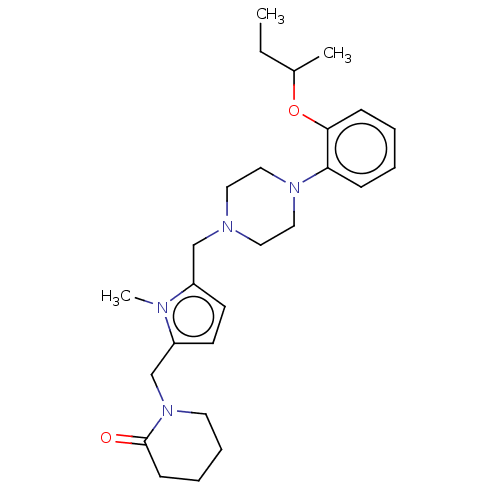 (1-{5-[4-(2-sec-Butoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214942 ((Z)-5-(4-hydroxybenzylidene)-10-methoxy-2,2,4-trim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415145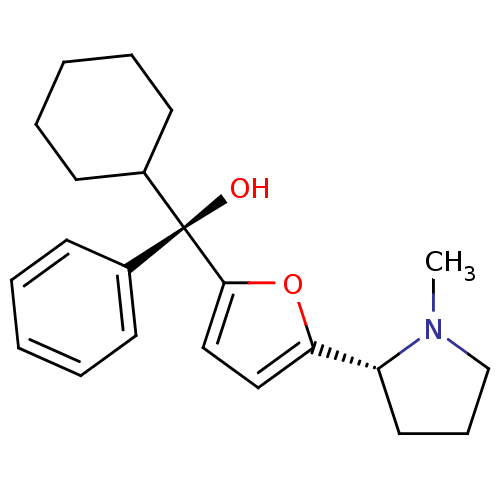 (CHEMBL571121) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50415144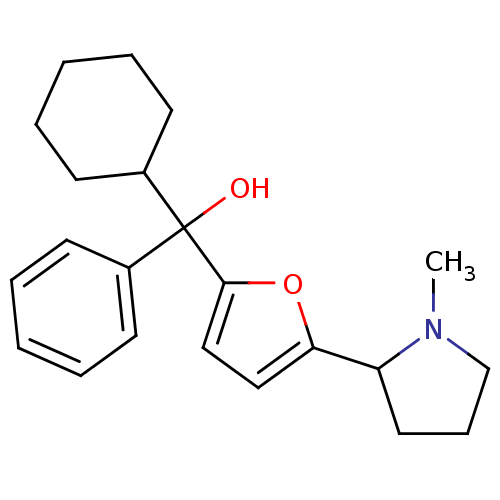 (CHEMBL583051) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 201-7 (2010) Article DOI: 10.1021/jm901048j BindingDB Entry DOI: 10.7270/Q2D79CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069021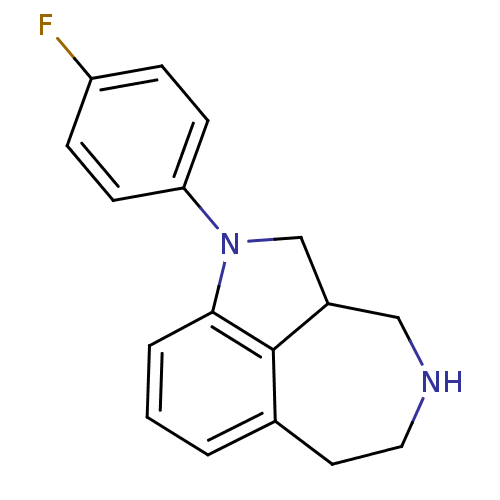 (1-(4-Fluoro-phenyl)-2,2a,3,4,5,6-hexahydro-1H-azep...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50377414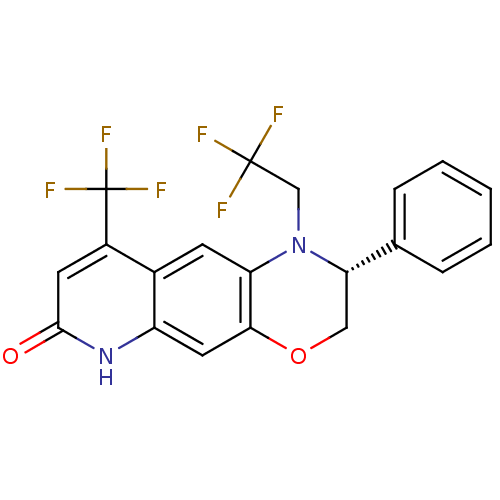 (CHEMBL402835) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 18: 2967-71 (2008) Article DOI: 10.1016/j.bmcl.2008.03.062 BindingDB Entry DOI: 10.7270/Q2R2128X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214922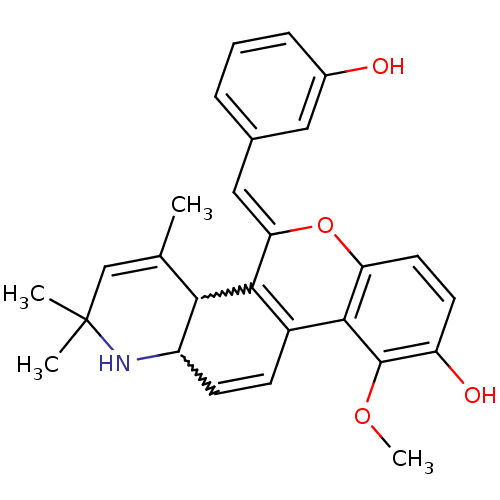 ((Z)-5-(3-hydroxybenzylidene)-10-methoxy-2,2,4-trim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001858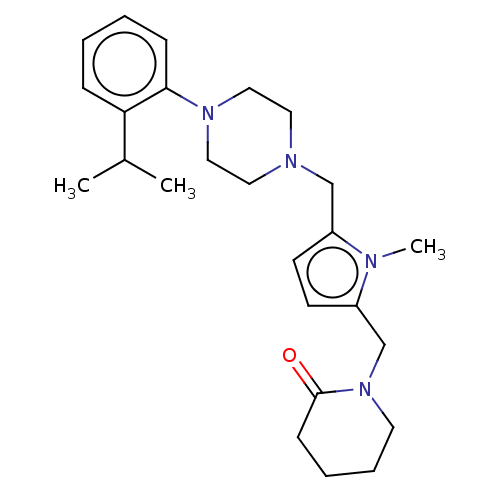 (1-{5-[4-(2-Isopropyl-phenyl)-piperazin-1-ylmethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214928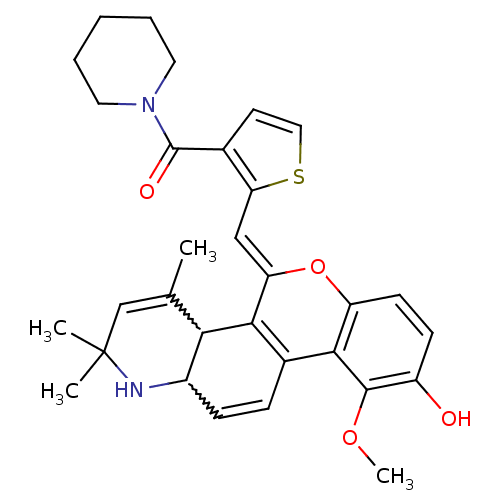 ((Z)-(2-((9-hydroxy-10-methoxy-2,2,4-trimethyl-1,2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from glucocorticoid receptor | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4376 total ) | Next | Last >> |