 Found 840 hits with Last Name = 'asso' and Initial = 'v'
Found 840 hits with Last Name = 'asso' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14028

((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(C)c12 |r| Show InChI InChI=1S/C16H21N3O2S/c1-12-9-18-11-14-5-3-6-15(16(12)14)22(20,21)19-8-4-7-17-10-13(19)2/h3,5-6,9,11,13,17H,4,7-8,10H2,1-2H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614318
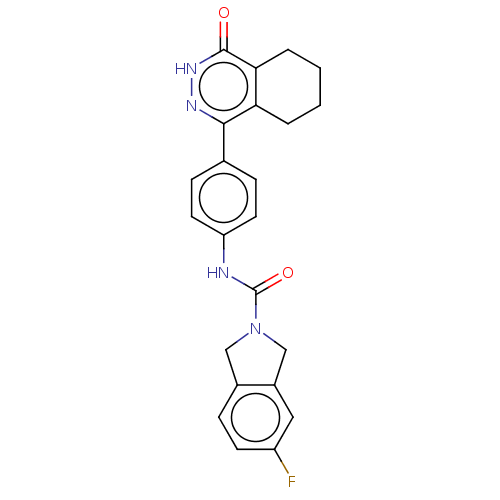
(CHEMBL5278707)Show SMILES Fc1ccc2CN(Cc2c1)C(=O)Nc1ccc(cc1)-c1n[nH]c(=O)c2CCCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM108025
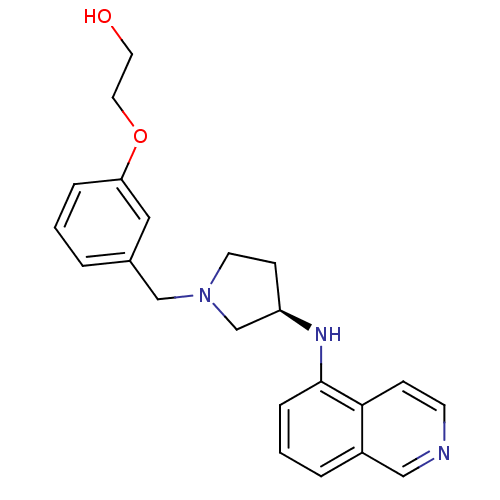
(US8604218, 2.039)Show SMILES OCCOc1cccc(CN2CC[C@H](C2)Nc2cccc3cnccc23)c1 |r| Show InChI InChI=1S/C22H25N3O2/c26-11-12-27-20-5-1-3-17(13-20)15-25-10-8-19(16-25)24-22-6-2-4-18-14-23-9-7-21(18)22/h1-7,9,13-14,19,24,26H,8,10-12,15-16H2/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614319
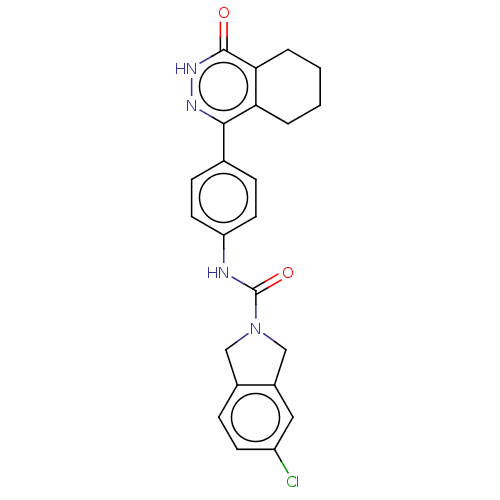
(CHEMBL5273210)Show SMILES Clc1ccc2CN(Cc2c1)C(=O)Nc1ccc(cc1)-c1n[nH]c(=O)c2CCCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50161159
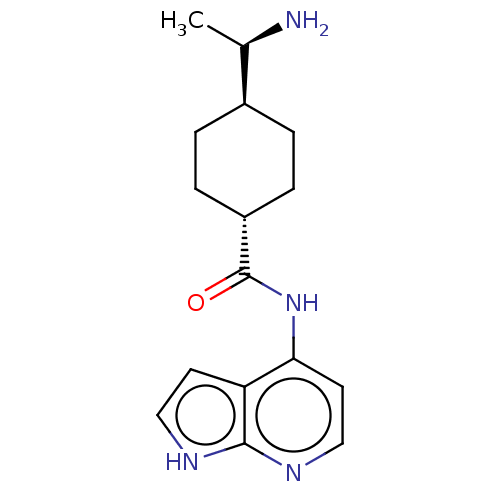
(CHEMBL3793353)Show SMILES [H][C@@]1(CC[C@@H](CC1)C(=O)Nc1ccnc2[nH]ccc12)[C@@H](C)N |r,wU:4.7,1.0,wD:19.23,(-1.43,8.98,;-2.33,8.49,;-3.67,7.73,;-3.68,6.19,;-2.35,5.41,;-1.01,6.17,;-1,7.71,;-2.35,3.87,;-3.42,3.26,;-1.02,3.09,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;-3.66,9.23,;-3.65,10.46,;-4.73,8.62,)| Show InChI InChI=1S/C18H18ClN3S/c1-22-8-7-12-9-14(19)16-17(21-18(23)20-16)15(12)13(10-22)11-5-3-2-4-6-11/h2-6,9,13H,7-8,10H2,1H3,(H2,20,21,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM108025

(US8604218, 2.039)Show SMILES OCCOc1cccc(CN2CC[C@H](C2)Nc2cccc3cnccc23)c1 |r| Show InChI InChI=1S/C22H25N3O2/c26-11-12-27-20-5-1-3-17(13-20)15-25-10-8-19(16-25)24-22-6-2-4-18-14-23-9-7-21(18)22/h1-7,9,13-14,19,24,26H,8,10-12,15-16H2/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM217082
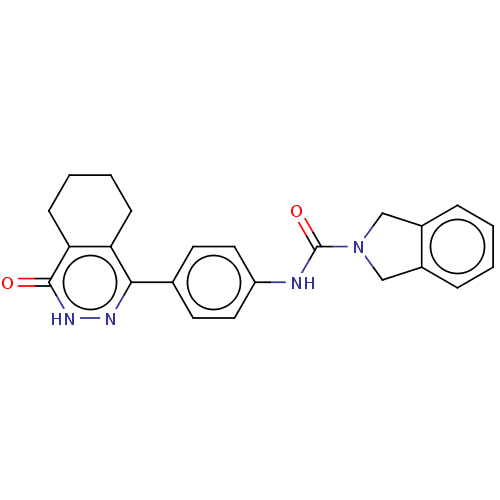
(US9302989, 377)Show SMILES O=C(Nc1ccc(cc1)-c1n[nH]c(=O)c2CCCCc12)N1Cc2ccccc2C1 Show InChI InChI=1S/C23H22N4O2/c28-22-20-8-4-3-7-19(20)21(25-26-22)15-9-11-18(12-10-15)24-23(29)27-13-16-5-1-2-6-17(16)14-27/h1-2,5-6,9-12H,3-4,7-8,13-14H2,(H,24,29)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50417857

(CHEMBL1667969)Show InChI InChI=1S/C14H16N2O2/c17-14-13-2-1-12(9-10(13)3-8-16-14)18-11-4-6-15-7-5-11/h1-3,8-9,11,15H,4-7H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14029
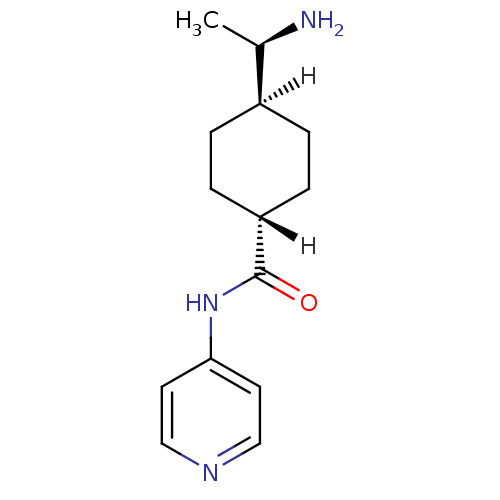
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14027
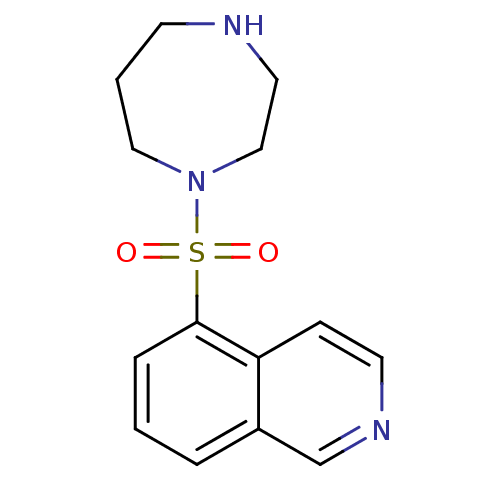
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485288
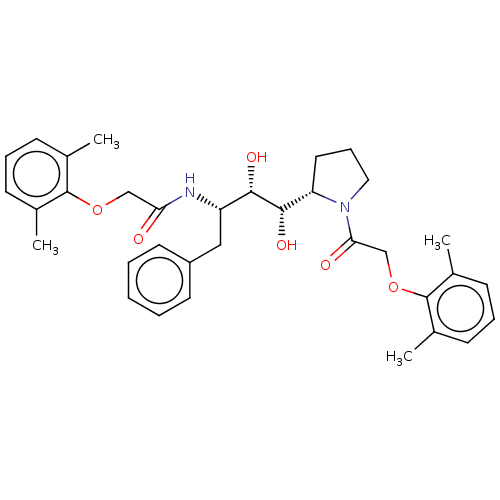
(CHEMBL2046980)Show SMILES [H][C@]1(CCCN1C(=O)COc1c(C)cccc1C)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C |r| Show InChI InChI=1S/C34H42N2O6/c1-22-11-8-12-23(2)33(22)41-20-29(37)35-27(19-26-15-6-5-7-16-26)31(39)32(40)28-17-10-18-36(28)30(38)21-42-34-24(3)13-9-14-25(34)4/h5-9,11-16,27-28,31-32,39-40H,10,17-21H2,1-4H3,(H,35,37)/t27-,28-,31-,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 3B protease activity in human H9 cells using Abz-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2 as substrate after 40 mins by spectrophotometr... |
J Med Chem 55: 3900-10 (2012)
Article DOI: 10.1021/jm3001136
BindingDB Entry DOI: 10.7270/Q2959MDC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16015
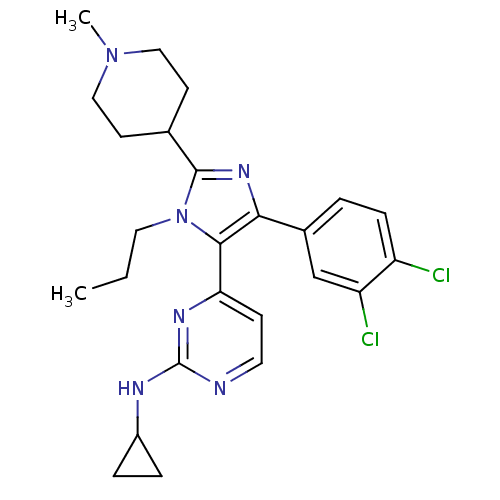
(CHEMBL252967 | N-cyclopropyl-4-[4-(3,4-dichlorophe...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCN(C)CC1 Show InChI InChI=1S/C25H30Cl2N6/c1-3-12-33-23(21-8-11-28-25(30-21)29-18-5-6-18)22(17-4-7-19(26)20(27)15-17)31-24(33)16-9-13-32(2)14-10-16/h4,7-8,11,15-16,18H,3,5-6,9-10,12-14H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories
| Assay Description
HTRF relies on fluorescence resonance energy transfer (FRET) between the donor, a europium cryptate (EuK), and the acceptor, the light harvesting pro... |
Chem Biol 10: 705-12 (2003)
Article DOI: 10.1016/S1074-5521(03)00159-5
BindingDB Entry DOI: 10.7270/Q2DJ5CWZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM86636
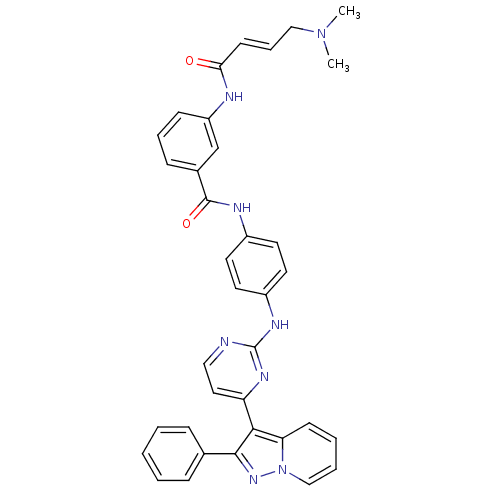
(JNK-IN-11)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C36H32N8O2/c1-43(2)22-9-15-32(45)38-29-13-8-12-26(24-29)35(46)39-27-16-18-28(19-17-27)40-36-37-21-20-30(41-36)33-31-14-6-7-23-44(31)42-34(33)25-10-4-3-5-11-25/h3-21,23-24H,22H2,1-2H3,(H,38,45)(H,39,46)(H,37,40,41)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86636

(JNK-IN-11)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C36H32N8O2/c1-43(2)22-9-15-32(45)38-29-13-8-12-26(24-29)35(46)39-27-16-18-28(19-17-27)40-36-37-21-20-30(41-36)33-31-14-6-7-23-44(31)42-34(33)25-10-4-3-5-11-25/h3-21,23-24H,22H2,1-2H3,(H,38,45)(H,39,46)(H,37,40,41)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86635
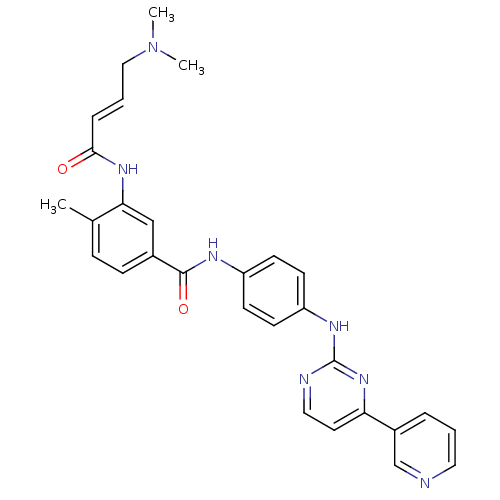
(JNK-IN-10)Show SMILES CN(C)C\C=C\C(=O)Nc1cc(ccc1C)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C29H29N7O2/c1-20-8-9-21(18-26(20)34-27(37)7-5-17-36(2)3)28(38)32-23-10-12-24(13-11-23)33-29-31-16-14-25(35-29)22-6-4-15-30-19-22/h4-16,18-19H,17H2,1-3H3,(H,32,38)(H,34,37)(H,31,33,35)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86634
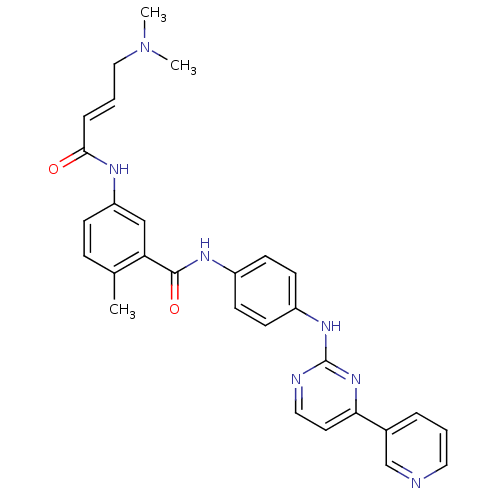
(JNK-IN-9)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(C)c(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C29H29N7O2/c1-20-8-9-24(32-27(37)7-5-17-36(2)3)18-25(20)28(38)33-22-10-12-23(13-11-22)34-29-31-16-14-26(35-29)21-6-4-15-30-19-21/h4-16,18-19H,17H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485278
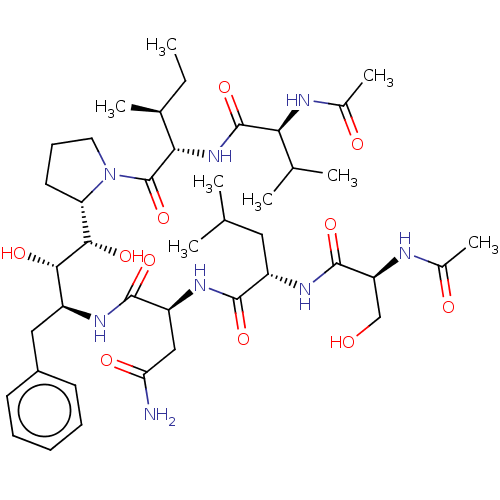
(CHEMBL2046922)Show SMILES [H][C@]1(CCCN1C(=O)[C@@H](NC(=O)[C@@H](NC(C)=O)C(C)C)[C@@H](C)CC)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(C)=O |r| Show InChI InChI=1S/C42H68N8O11/c1-9-24(6)35(49-41(60)34(23(4)5)45-26(8)53)42(61)50-17-13-16-32(50)37(56)36(55)28(19-27-14-11-10-12-15-27)46-39(58)30(20-33(43)54)48-38(57)29(18-22(2)3)47-40(59)31(21-51)44-25(7)52/h10-12,14-15,22-24,28-32,34-37,51,55-56H,9,13,16-21H2,1-8H3,(H2,43,54)(H,44,52)(H,45,53)(H,46,58)(H,47,59)(H,48,57)(H,49,60)/t24-,28-,29-,30-,31-,32-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease using Abz-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2 as substrate by fluorescence analysis |
J Med Chem 55: 3900-10 (2012)
Article DOI: 10.1021/jm3001136
BindingDB Entry DOI: 10.7270/Q2959MDC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485285
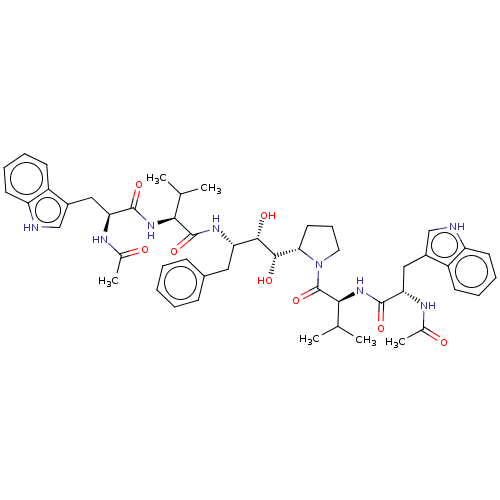
(CHEMBL1233644)Show SMILES [H][C@]1(CCCN1C(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(C)C)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(C)C |r| Show InChI InChI=1S/C50H64N8O8/c1-28(2)43(56-47(63)40(53-30(5)59)24-33-26-51-37-19-12-10-17-35(33)37)49(65)55-39(23-32-15-8-7-9-16-32)45(61)46(62)42-21-14-22-58(42)50(66)44(29(3)4)57-48(64)41(54-31(6)60)25-34-27-52-38-20-13-11-18-36(34)38/h7-13,15-20,26-29,39-46,51-52,61-62H,14,21-25H2,1-6H3,(H,53,59)(H,54,60)(H,55,65)(H,56,63)(H,57,64)/t39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease using Abz-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2 as substrate by fluorescence analysis |
J Med Chem 55: 3900-10 (2012)
Article DOI: 10.1021/jm3001136
BindingDB Entry DOI: 10.7270/Q2959MDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86632
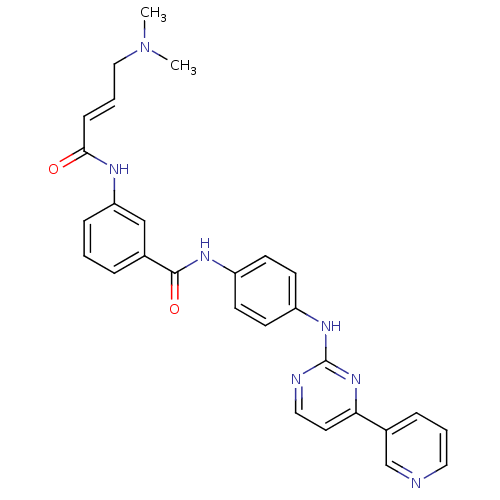
(JNK-IN-7)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C28H27N7O2/c1-35(2)17-5-9-26(36)31-24-8-3-6-20(18-24)27(37)32-22-10-12-23(13-11-22)33-28-30-16-14-25(34-28)21-7-4-15-29-19-21/h3-16,18-19H,17H2,1-2H3,(H,31,36)(H,32,37)(H,30,33,34)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86630
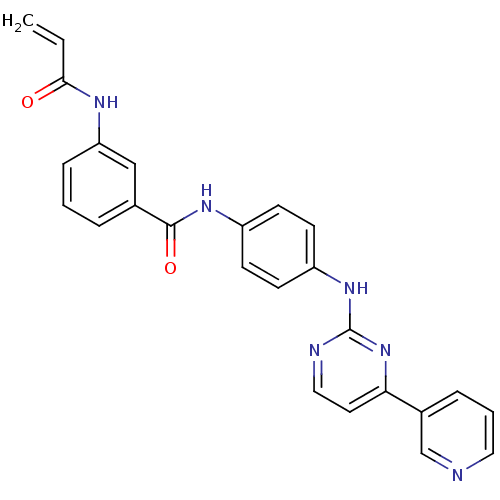
(JNK-IN-5)Show SMILES C=CC(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C25H20N6O2/c1-2-23(32)28-21-7-3-5-17(15-21)24(33)29-19-8-10-20(11-9-19)30-25-27-14-12-22(31-25)18-6-4-13-26-16-18/h2-16H,1H2,(H,28,32)(H,29,33)(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM86633
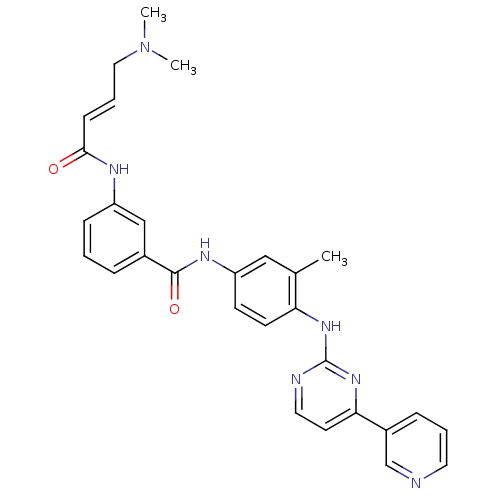
(JNK-IN-8)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)c(C)c1 Show InChI InChI=1S/C29H29N7O2/c1-20-17-24(11-12-25(20)34-29-31-15-13-26(35-29)22-8-5-14-30-19-22)33-28(38)21-7-4-9-23(18-21)32-27(37)10-6-16-36(2)3/h4-15,17-19H,16H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50089582
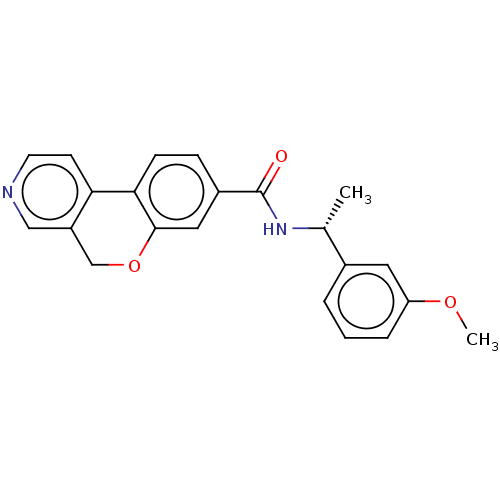
(CHEMBL3578250 | US9914740, I-1)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc-2c(OCc3cnccc-23)c1 |r| Show InChI InChI=1S/C22H20N2O3/c1-14(15-4-3-5-18(10-15)26-2)24-22(25)16-6-7-20-19-8-9-23-12-17(19)13-27-21(20)11-16/h3-12,14H,13H2,1-2H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50430374
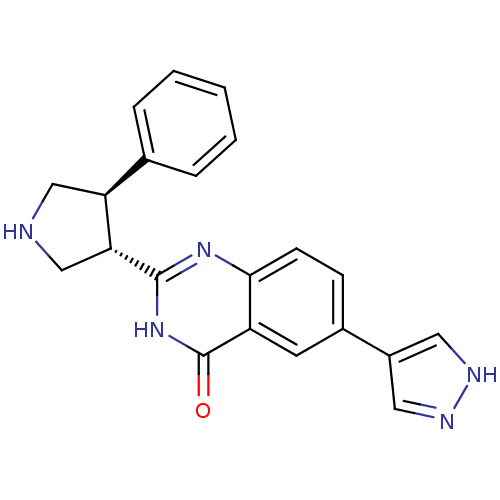
(CHEMBL2334292)Show SMILES O=c1[nH]c(nc2ccc(cc12)-c1cn[nH]c1)[C@@H]1CNC[C@H]1c1ccccc1 |r| Show InChI InChI=1S/C21H19N5O/c27-21-16-8-14(15-9-23-24-10-15)6-7-19(16)25-20(26-21)18-12-22-11-17(18)13-4-2-1-3-5-13/h1-10,17-18,22H,11-12H2,(H,23,24)(H,25,26,27)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 6-His-tagged ROCK2 expressed in baculovirus-infected Sf9 cells using LCB-AKRRRLSSLRA-NH2 as substrate after 25 mins b... |
Bioorg Med Chem Lett 23: 1592-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.109
BindingDB Entry DOI: 10.7270/Q2TD9ZQM |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM412136

(US10392402, Compound A | US10738007, Compound A | ...)Show SMILES Cc1c[nH]c2nccc(Oc3c(F)cc(Nc4cc(Cl)nc(N)n4)cc3F)c12 Show InChI InChI=1S/C18H13ClF2N6O/c1-8-7-24-17-15(8)12(2-3-23-17)28-16-10(20)4-9(5-11(16)21)25-14-6-13(19)26-18(22)27-14/h2-7H,1H3,(H,23,24)(H3,22,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614301
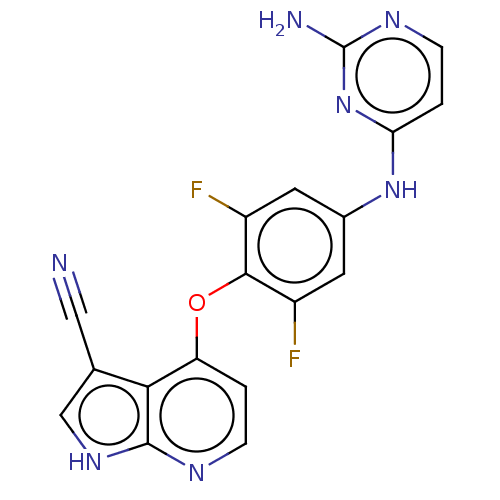
(CHEMBL5270382)Show SMILES Nc1nccc(Nc2cc(F)c(Oc3ccnc4[nH]cc(C#N)c34)c(F)c2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614300
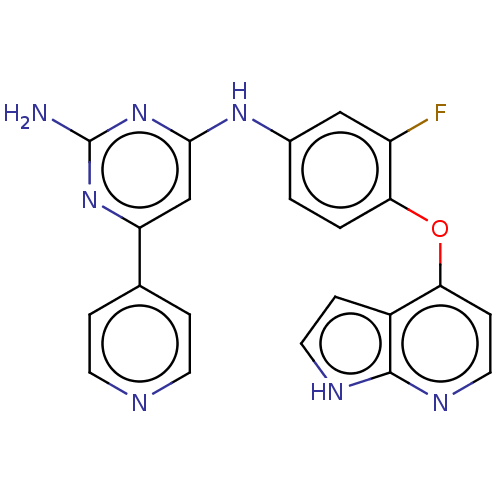
(CHEMBL5274003)Show SMILES Nc1nc(Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)cc(n1)-c1ccncc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM252001

(US9458110, 1)Show SMILES COc1cccc(CNC(=O)c2ccc(-c3cn[nH]c3)c(OC)c2)c1 Show InChI InChI=1S/C19H19N3O3/c1-24-16-5-3-4-13(8-16)10-20-19(23)14-6-7-17(18(9-14)25-2)15-11-21-22-12-15/h3-9,11-12H,10H2,1-2H3,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM251935

(US9458110, 7)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(-c2cn[nH]c2)c(OC)c1 Show InChI InChI=1S/C20H21N3O3/c1-13(14-5-4-6-17(9-14)25-2)23-20(24)15-7-8-18(19(10-15)26-3)16-11-21-22-12-16/h4-13H,1-3H3,(H,21,22)(H,23,24)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
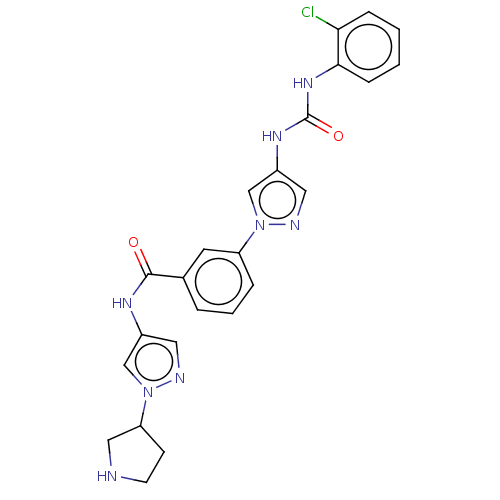
(Homo sapiens (Human)) | BDBM50032821
(CHEMBL3355178)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)Nc1cnn(c1)C1CCNC1 Show InChI InChI=1S/C24H23ClN8O2/c25-21-6-1-2-7-22(21)31-24(35)30-18-12-27-32(15-18)19-5-3-4-16(10-19)23(34)29-17-11-28-33(14-17)20-8-9-26-13-20/h1-7,10-12,14-15,20,26H,8-9,13H2,(H,29,34)(H2,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431137
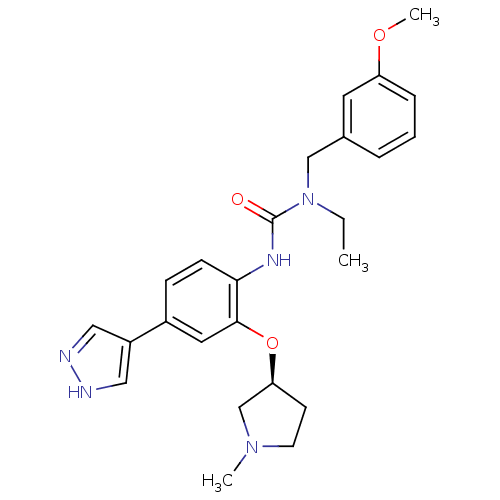
(CHEMBL2332060)Show SMILES CCN(Cc1cccc(OC)c1)C(=O)Nc1ccc(cc1O[C@H]1CCN(C)C1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C25H31N5O3/c1-4-30(16-18-6-5-7-21(12-18)32-3)25(31)28-23-9-8-19(20-14-26-27-15-20)13-24(23)33-22-10-11-29(2)17-22/h5-9,12-15,22H,4,10-11,16-17H2,1-3H3,(H,26,27)(H,28,31)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431143
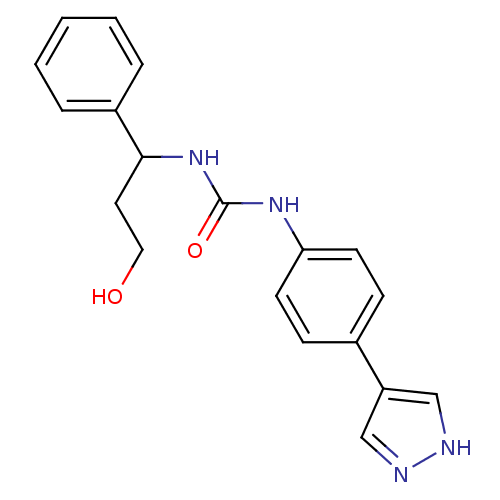
(CHEMBL2332057)Show InChI InChI=1S/C19H20N4O2/c24-11-10-18(15-4-2-1-3-5-15)23-19(25)22-17-8-6-14(7-9-17)16-12-20-21-13-16/h1-9,12-13,18,24H,10-11H2,(H,20,21)(H2,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431134
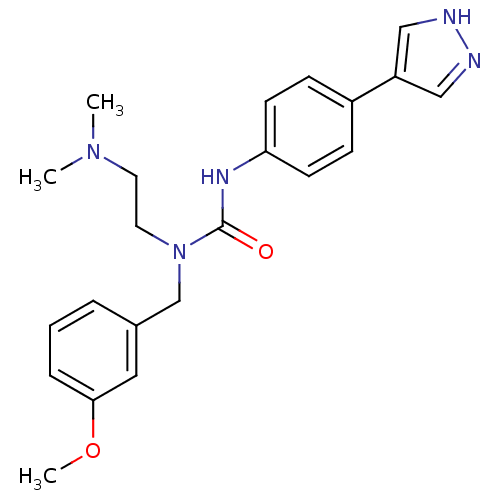
(CHEMBL2332068)Show SMILES COc1cccc(CN(CCN(C)C)C(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C22H27N5O2/c1-26(2)11-12-27(16-17-5-4-6-21(13-17)29-3)22(28)25-20-9-7-18(8-10-20)19-14-23-24-15-19/h4-10,13-15H,11-12,16H2,1-3H3,(H,23,24)(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
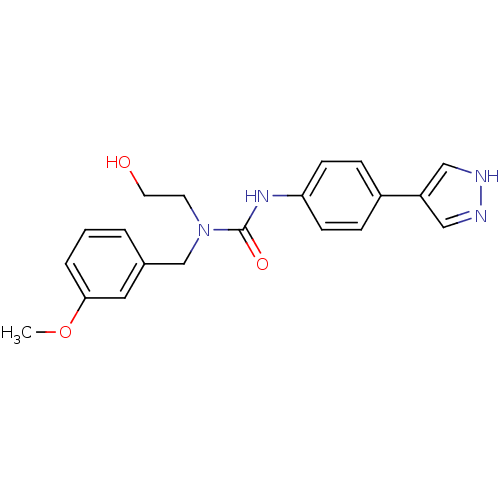
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431135
(CHEMBL2332065)Show SMILES COc1cccc(CN(CCO)C(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H22N4O3/c1-27-19-4-2-3-15(11-19)14-24(9-10-25)20(26)23-18-7-5-16(6-8-18)17-12-21-22-13-17/h2-8,11-13,25H,9-10,14H2,1H3,(H,21,22)(H,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
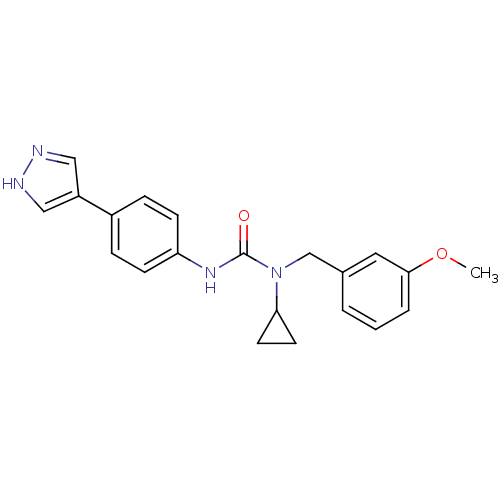
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431144
(CHEMBL2332099)Show SMILES COc1cccc(CN(C2CC2)C(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C21H22N4O2/c1-27-20-4-2-3-15(11-20)14-25(19-9-10-19)21(26)24-18-7-5-16(6-8-18)17-12-22-23-13-17/h2-8,11-13,19H,9-10,14H2,1H3,(H,22,23)(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
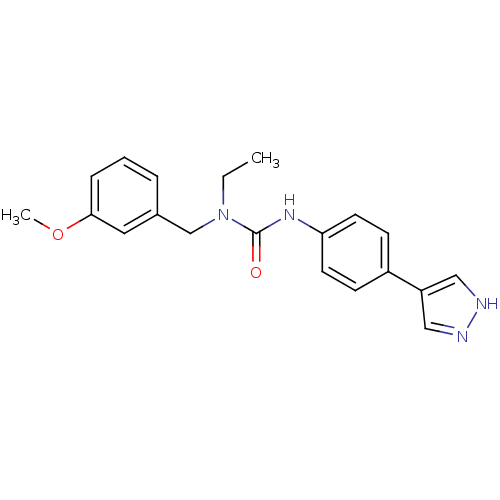
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431145
(CHEMBL2332098)Show SMILES CCN(Cc1cccc(OC)c1)C(=O)Nc1ccc(cc1)-c1cn[nH]c1 Show InChI InChI=1S/C20H22N4O2/c1-3-24(14-15-5-4-6-19(11-15)26-2)20(25)23-18-9-7-16(8-10-18)17-12-21-22-13-17/h4-13H,3,14H2,1-2H3,(H,21,22)(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50431138
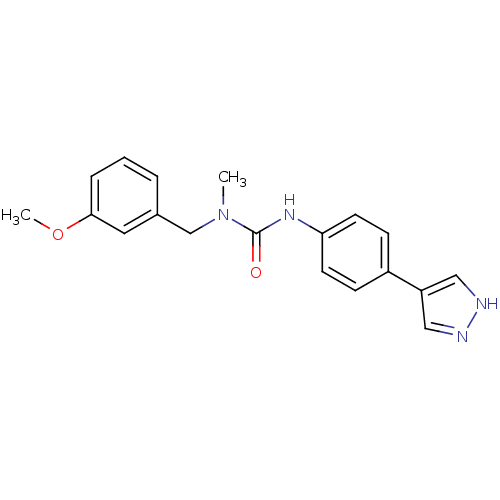
(CHEMBL2332097)Show SMILES COc1cccc(CN(C)C(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C19H20N4O2/c1-23(13-14-4-3-5-18(10-14)25-2)19(24)22-17-8-6-15(7-9-17)16-11-20-21-12-16/h3-12H,13H2,1-2H3,(H,20,21)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) after 4 hrs by HTRF assay |
J Med Chem 56: 3568-81 (2013)
Article DOI: 10.1021/jm400062r
BindingDB Entry DOI: 10.7270/Q28C9XMJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM251935

(US9458110, 7)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(-c2cn[nH]c2)c(OC)c1 Show InChI InChI=1S/C20H21N3O3/c1-13(14-5-4-6-17(9-14)25-2)23-20(24)15-7-8-18(19(10-15)26-3)16-11-21-22-12-16/h4-13H,1-3H3,(H,21,22)(H,23,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50430377
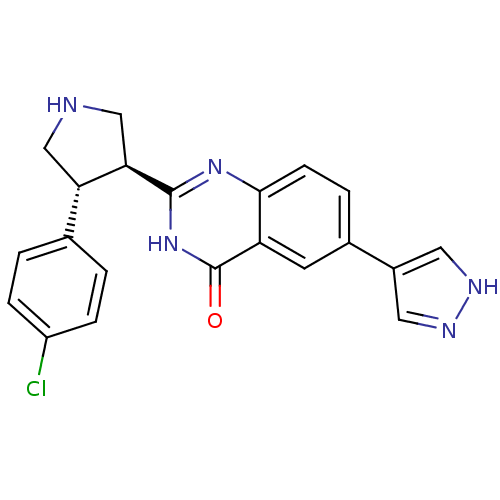
(CHEMBL2333898)Show SMILES Clc1ccc(cc1)[C@@H]1CNC[C@H]1c1nc2ccc(cc2c(=O)[nH]1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C21H18ClN5O/c22-15-4-1-12(2-5-15)17-10-23-11-18(17)20-26-19-6-3-13(14-8-24-25-9-14)7-16(19)21(28)27-20/h1-9,17-18,23H,10-11H2,(H,24,25)(H,26,27,28)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Bioorg Med Chem Lett 23: 1592-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.109
BindingDB Entry DOI: 10.7270/Q2TD9ZQM |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614310
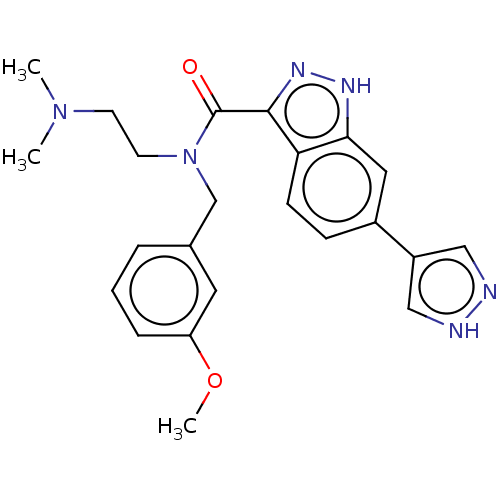
(CHEMBL5269663)Show SMILES COc1cccc(CN(CCN(C)C)C(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614312

(CHEMBL5284365)Show SMILES COc1cccc(CNC(=O)c2nn(C)c3cc(ccc23)-c2cn[nH]c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50614313
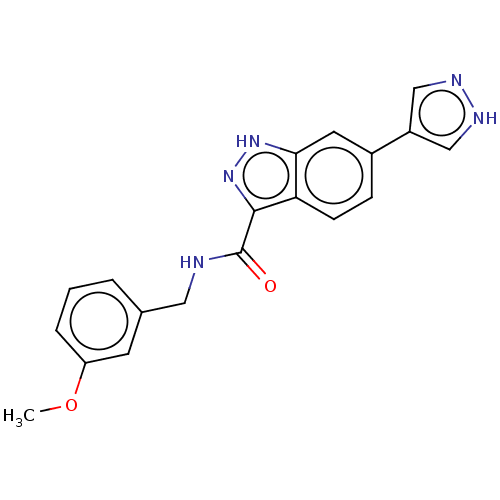
(CHEMBL5283378)Show SMILES COc1cccc(CNC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50026615
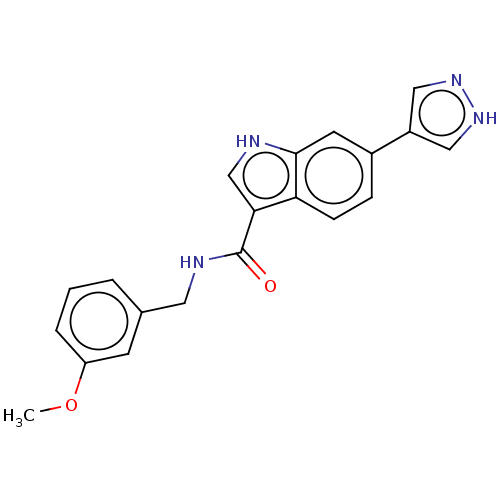
(CHEMBL1922045)Show SMILES COc1cccc(CNC(=O)c2c[nH]c3cc(ccc23)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H18N4O2/c1-26-16-4-2-3-13(7-16)9-22-20(25)18-12-21-19-8-14(5-6-17(18)19)15-10-23-24-11-15/h2-8,10-12,21H,9H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50495031

(CHEMBL3099975)Show SMILES Oc1onc(c1-c1ccc(F)cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)c1 Show InChI InChI=1S/C24H21FN4O3/c25-18-3-1-16(2-4-18)22-23(28-32-24(22)30)17-9-10-26-21(15-17)27-19-5-7-20(8-6-19)29-11-13-31-14-12-29/h1-10,15,30H,11-14H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (39 to 402 amino acids) (unknown origin) expressed in Escherichia coli BL21(DE3) using biotinylated ATF2 as substrate after 15 min... |
Bioorg Med Chem Lett 24: 161-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.052
BindingDB Entry DOI: 10.7270/Q2XG9V3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM86636

(JNK-IN-11)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C36H32N8O2/c1-43(2)22-9-15-32(45)38-29-13-8-12-26(24-29)35(46)39-27-16-18-28(19-17-27)40-36-37-21-20-30(41-36)33-31-14-6-7-23-44(31)42-34(33)25-10-4-3-5-11-25/h3-21,23-24H,22H2,1-2H3,(H,38,45)(H,39,46)(H,37,40,41)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM86632

(JNK-IN-7)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C28H27N7O2/c1-35(2)17-5-9-26(36)31-24-8-3-6-20(18-24)27(37)32-22-10-12-23(13-11-22)33-28-30-16-14-25(34-28)21-7-4-15-29-19-21/h3-16,18-19H,17H2,1-2H3,(H,31,36)(H,32,37)(H,30,33,34)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM16016
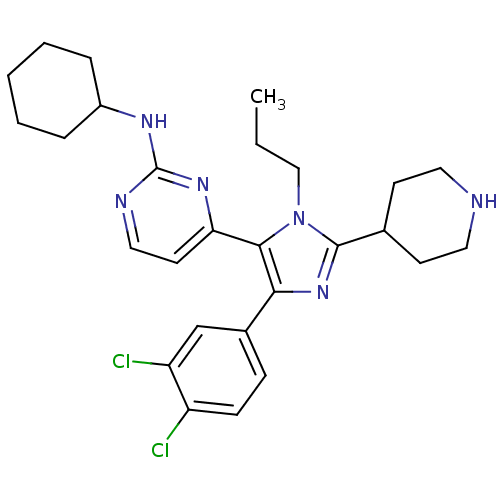
(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories
| Assay Description
HTRF relies on fluorescence resonance energy transfer (FRET) between the donor, a europium cryptate (EuK), and the acceptor, the light harvesting pro... |
Chem Biol 10: 705-12 (2003)
Article DOI: 10.1016/S1074-5521(03)00159-5
BindingDB Entry DOI: 10.7270/Q2DJ5CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50358469
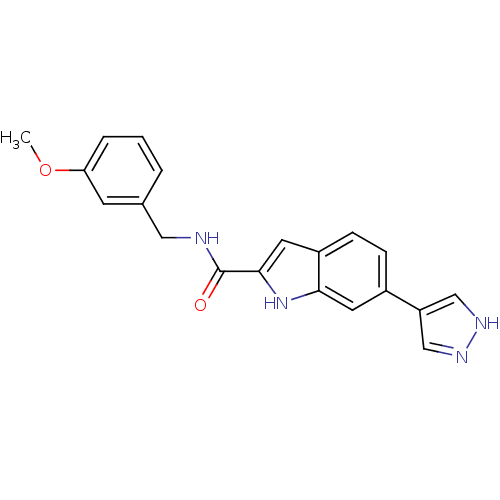
(CHEMBL1922026)Show SMILES COc1cccc(CNC(=O)c2cc3ccc(cc3[nH]2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H18N4O2/c1-26-17-4-2-3-13(7-17)10-21-20(25)19-9-15-6-5-14(8-18(15)24-19)16-11-22-23-12-16/h2-9,11-12,24H,10H2,1H3,(H,21,25)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485287
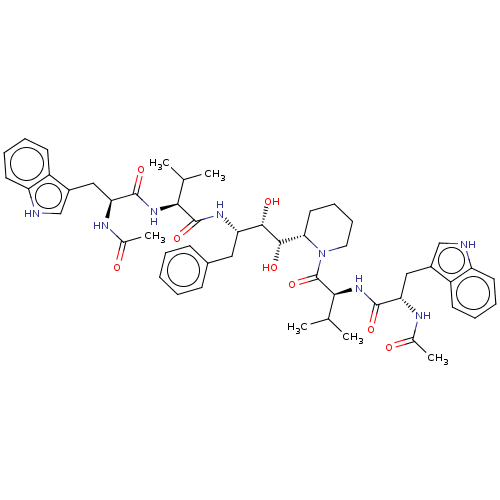
(CHEMBL2046975)Show SMILES [H][C@]1(CCCCN1C(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(C)C)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(C)C |r| Show InChI InChI=1S/C51H66N8O8/c1-29(2)44(57-48(64)41(54-31(5)60)25-34-27-52-38-20-12-10-18-36(34)38)50(66)56-40(24-33-16-8-7-9-17-33)46(62)47(63)43-22-14-15-23-59(43)51(67)45(30(3)4)58-49(65)42(55-32(6)61)26-35-28-53-39-21-13-11-19-37(35)39/h7-13,16-21,27-30,40-47,52-53,62-63H,14-15,22-26H2,1-6H3,(H,54,60)(H,55,61)(H,56,66)(H,57,64)(H,58,65)/t40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 protease using Abz-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2 as substrate by fluorescence analysis |
J Med Chem 55: 3900-10 (2012)
Article DOI: 10.1021/jm3001136
BindingDB Entry DOI: 10.7270/Q2959MDC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM86630

(JNK-IN-5)Show SMILES C=CC(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C25H20N6O2/c1-2-23(32)28-21-7-3-5-17(15-21)24(33)29-19-8-10-20(11-9-19)30-25-27-14-12-22(31-25)18-6-4-13-26-16-18/h2-16H,1H2,(H,28,32)(H,29,33)(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM86632

(JNK-IN-7)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)C(=O)Nc1ccc(Nc2nccc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C28H27N7O2/c1-35(2)17-5-9-26(36)31-24-8-3-6-20(18-24)27(37)32-22-10-12-23(13-11-22)33-28-30-16-14-25(34-28)21-7-4-15-29-19-21/h3-16,18-19H,17H2,1-2H3,(H,31,36)(H,32,37)(H,30,33,34)/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data