

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50155838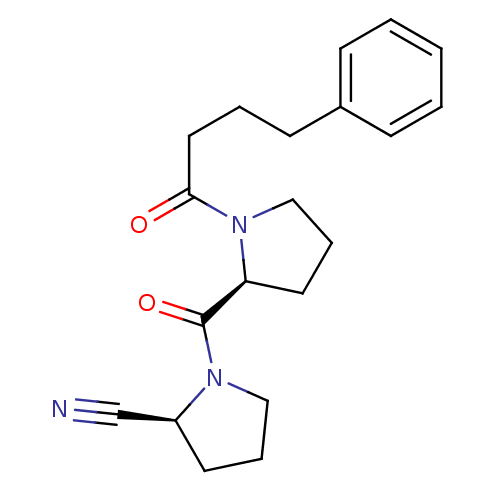 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of POP in Han/Wistar rat brain using Suc-Gly-Pro-AMC substrate incubated for 60 mins | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50051495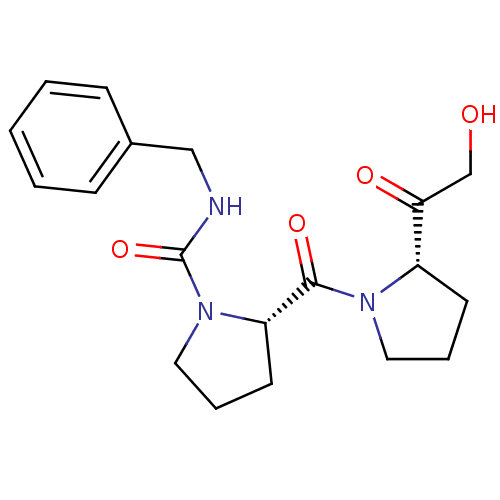 ((S)-2-[(S)-2-(2-Hydroxy-acetyl)-pyrrolidine-1-carb...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of poricne brain POP in expressed in Escherichia coli TOP10 competent cells pre-incubated for 2 hrs before Z-Gly-Pro-AMC substrate additio... | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Bos taurus) | BDBM50038879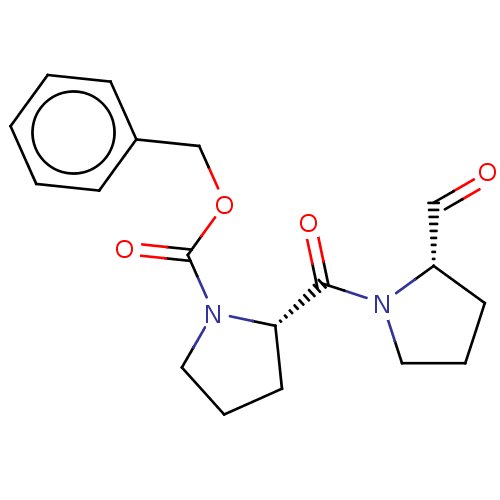 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of POP in bovine serum using Z-Gly-Pro-NH-Mec fluorimetric substrate | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180679 (CHEMBL3817958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP by tight binding based Morrison equation analysis | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50155838 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP by tight binding based Morrison equation analysis | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50155838 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180679 (CHEMBL3817958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193475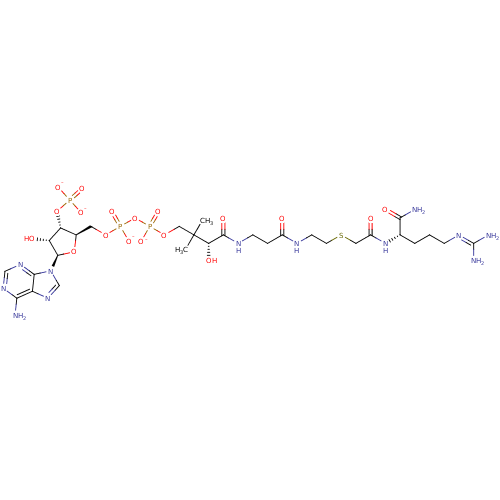 ((3R)-3-{[2-({2-[({[(1S)-1-carbamoyl-4-[(diaminomet...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818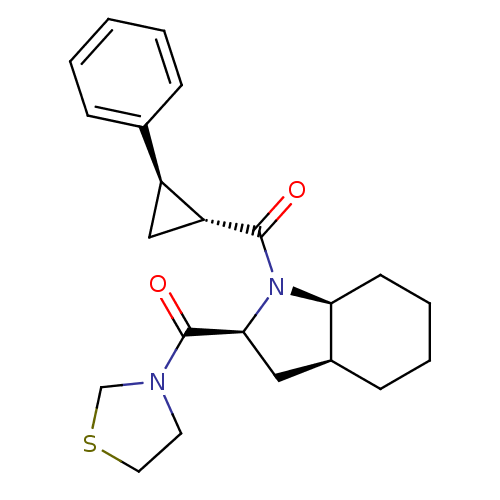 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP using Z-Gly-Pro-7-AMC substrate | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193477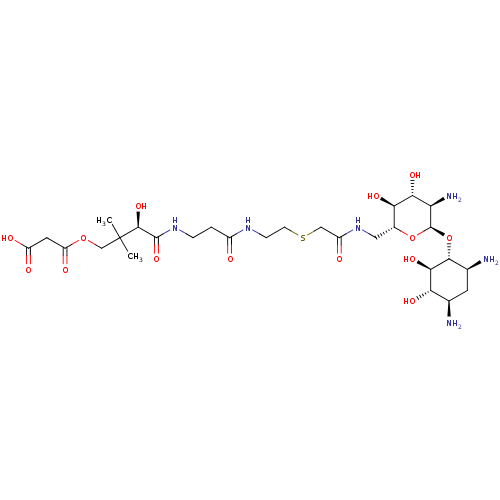 (3-((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193487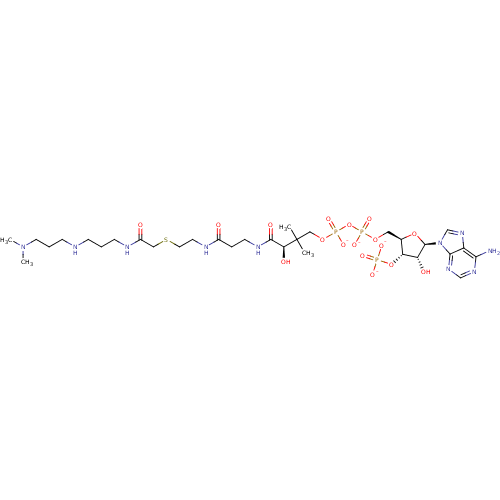 ((3R)-3-[(2-{[2-({[(3-{[3-(dimethylamino)propyl]ami...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193484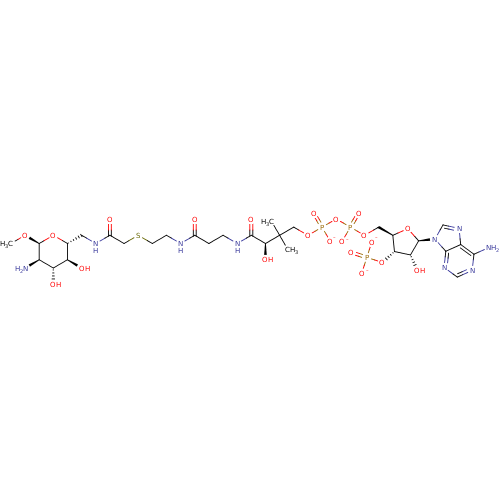 (CID44414951 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193478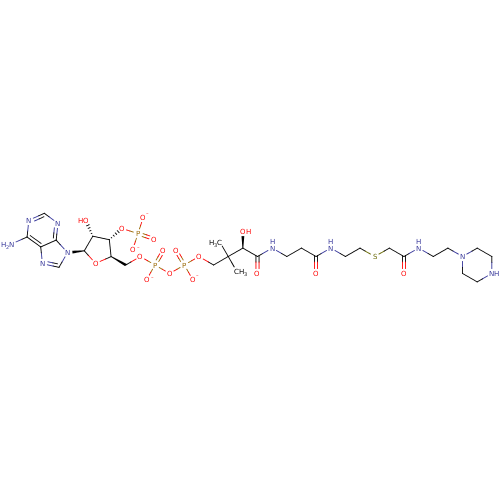 (CID44415032 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193488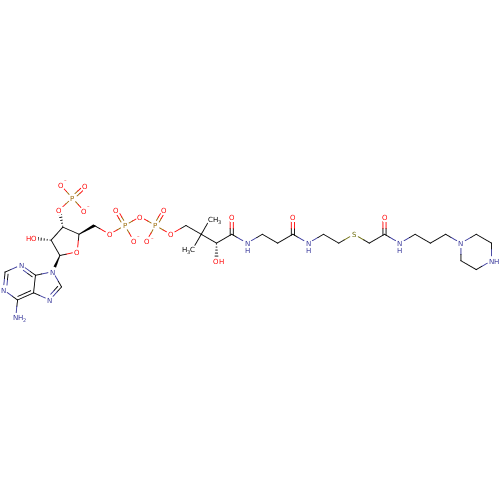 (CID44415033 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180664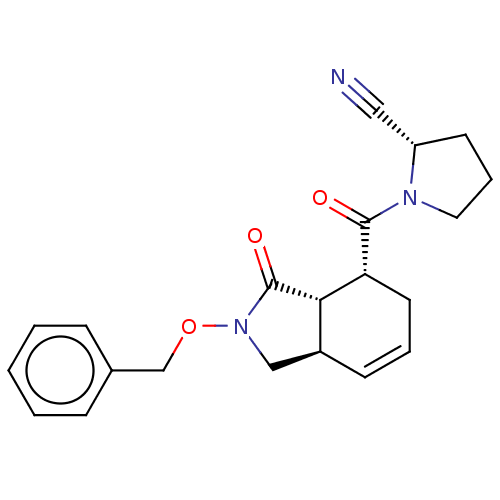 (CHEMBL3818013) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193474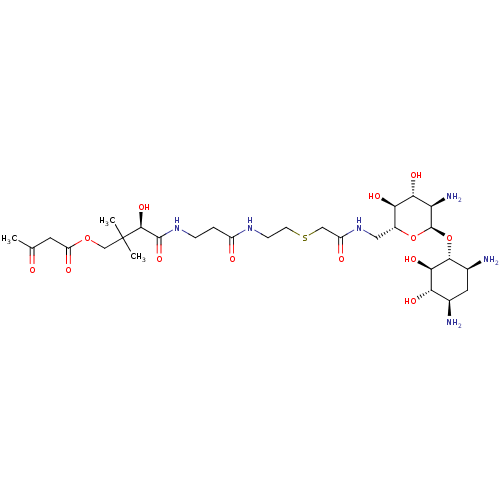 ((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193480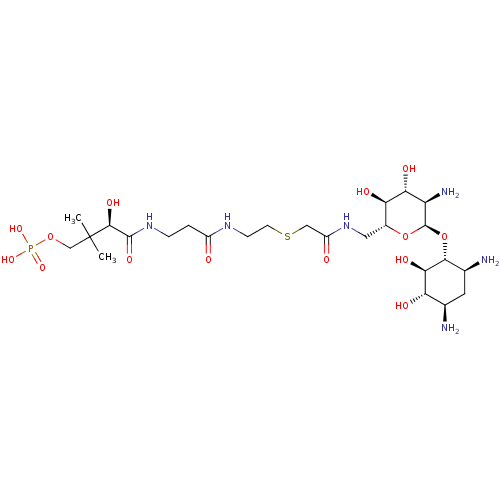 ((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50393843 (CHEMBL2159748) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180669 (CHEMBL3818020) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193476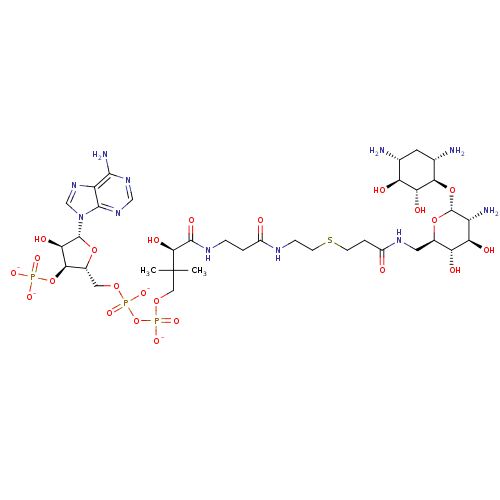 (CID44415023 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180678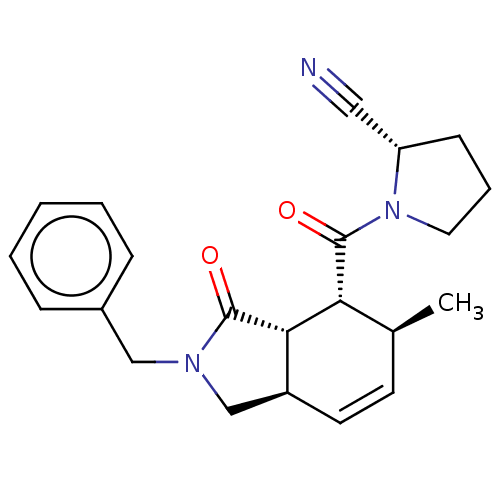 (CHEMBL3819136) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193486 (CID44415022 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193482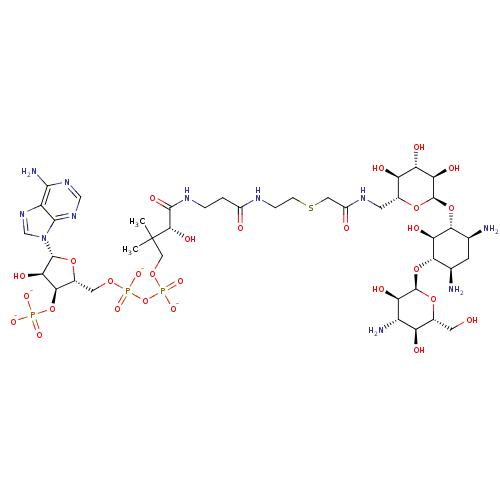 (CID44414946 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193479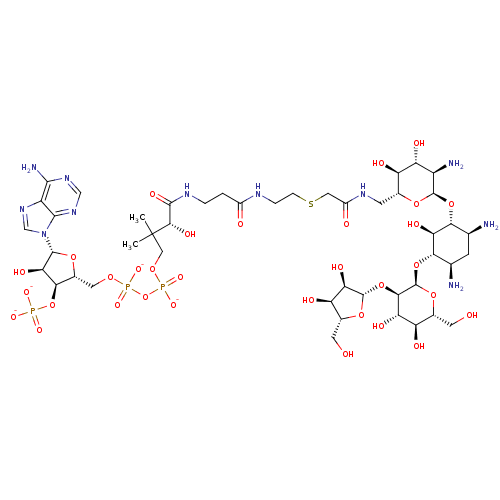 (CID44414949 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193485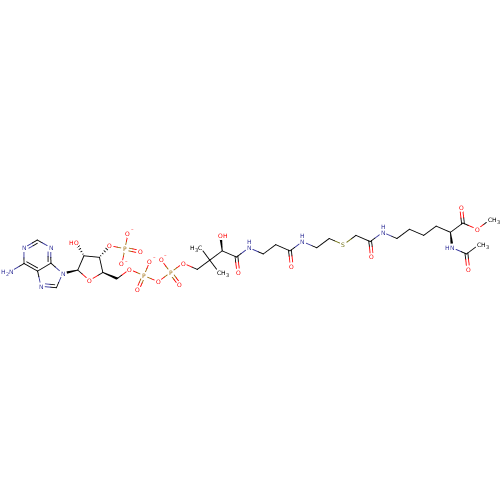 ((3R)-3-{[2-({2-[({[(5S)-5-acetamido-6-methoxy-6-ox...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193483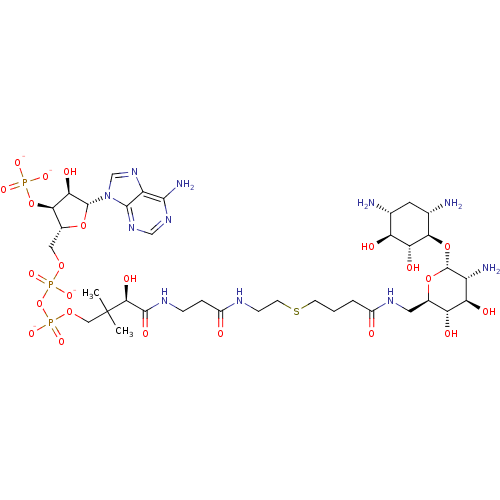 (CID44415024 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180668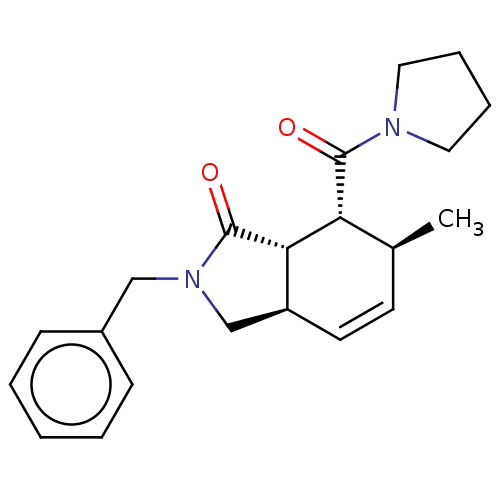 (CHEMBL3819096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180667 (CHEMBL3818815) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180681 (CHEMBL3818902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180665 (CHEMBL3819346) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180670 (CHEMBL3819362) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193481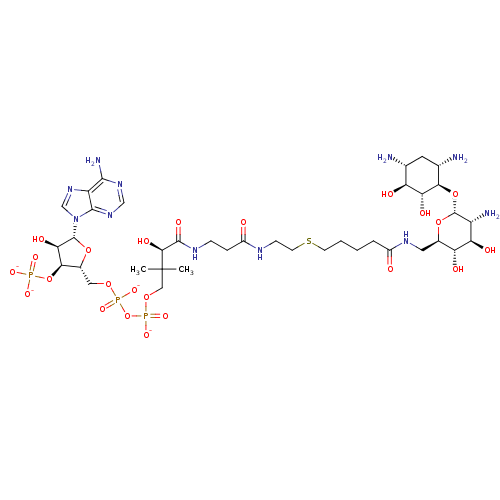 (CID44414945 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180673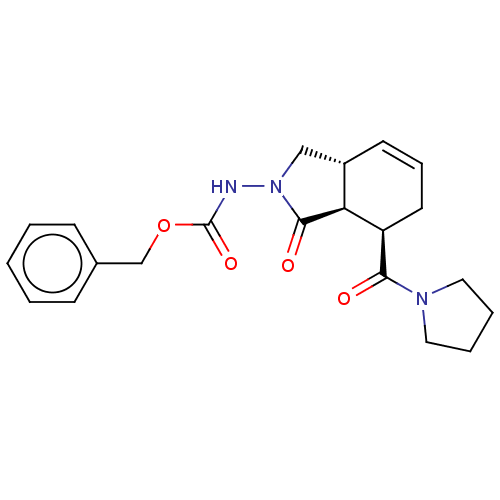 (CHEMBL3819408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180680 (CHEMBL3818446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180666 (CHEMBL3818472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180671 (CHEMBL3819072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180672 (CHEMBL3818541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180677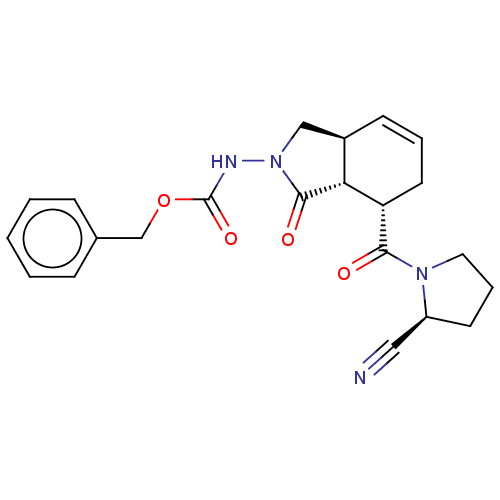 (CHEMBL3818999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180674 (CHEMBL3818697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180682 (CHEMBL3819233) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180676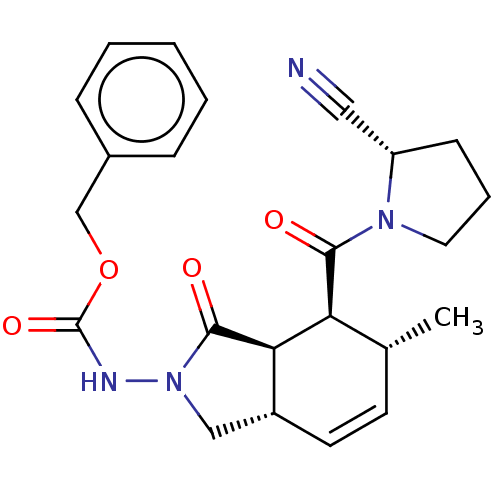 (CHEMBL3818703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180675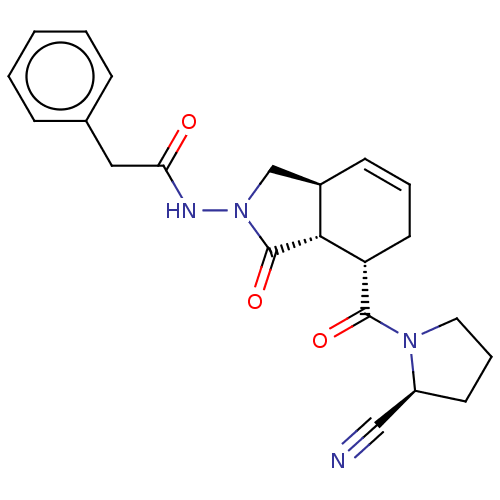 (CHEMBL3819297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type III pantothenate kinase (Bacillus anthracis) | BDBM50466686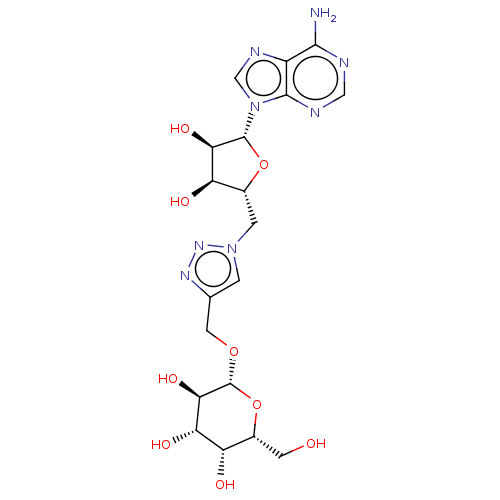 (CHEMBL4292402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis pantothenate kinase 3 | Bioorg Med Chem 26: 5896-5902 (2018) Article DOI: 10.1016/j.bmc.2018.10.042 BindingDB Entry DOI: 10.7270/Q2251MWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50312223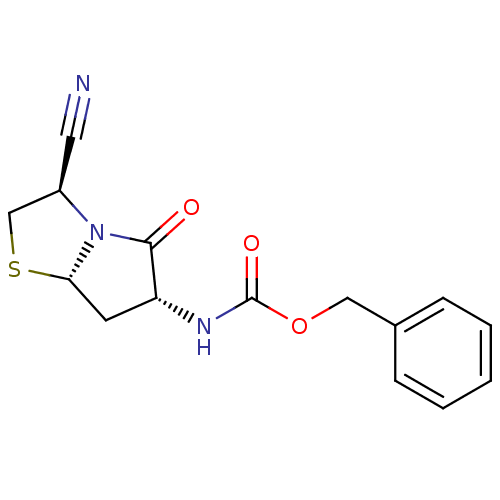 ((2R,5S,7R)-1-Aza-7-benzyloxycarbonylamino-8-oxo-4-...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of poricne POP in cell lysates incubated for 60 mins using Z-Gly-Pro-AMC as a substrate by fluorescence assay | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50312223 ((2R,5S,7R)-1-Aza-7-benzyloxycarbonylamino-8-oxo-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) in living cells | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM92669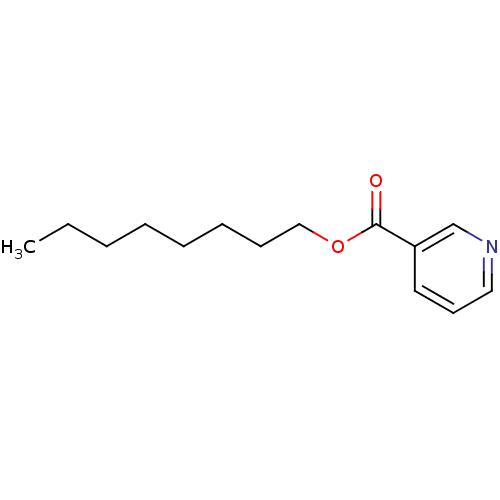 (Auxiliary substrate, 14a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University | Assay Description All UV/Vis spectra of the enzyme-ligand complexes were obtained on a Cary 5000 UV/Vis spectrophotometer. | Chembiochem 13: 2527-36 (2012) Article DOI: 10.1002/cbic.201200524 BindingDB Entry DOI: 10.7270/Q2PR7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM92667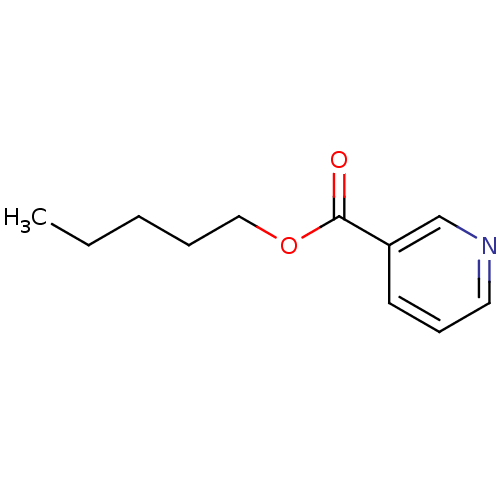 (Auxiliary substrate, 12a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University | Assay Description All UV/Vis spectra of the enzyme-ligand complexes were obtained on a Cary 5000 UV/Vis spectrophotometer. | Chembiochem 13: 2527-36 (2012) Article DOI: 10.1002/cbic.201200524 BindingDB Entry DOI: 10.7270/Q2PR7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM92666 (Auxiliary substrate, 11a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University | Assay Description All UV/Vis spectra of the enzyme-ligand complexes were obtained on a Cary 5000 UV/Vis spectrophotometer. | Chembiochem 13: 2527-36 (2012) Article DOI: 10.1002/cbic.201200524 BindingDB Entry DOI: 10.7270/Q2PR7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM92665 (Auxiliary substrate, 10a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University | Assay Description All UV/Vis spectra of the enzyme-ligand complexes were obtained on a Cary 5000 UV/Vis spectrophotometer. | Chembiochem 13: 2527-36 (2012) Article DOI: 10.1002/cbic.201200524 BindingDB Entry DOI: 10.7270/Q2PR7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM92670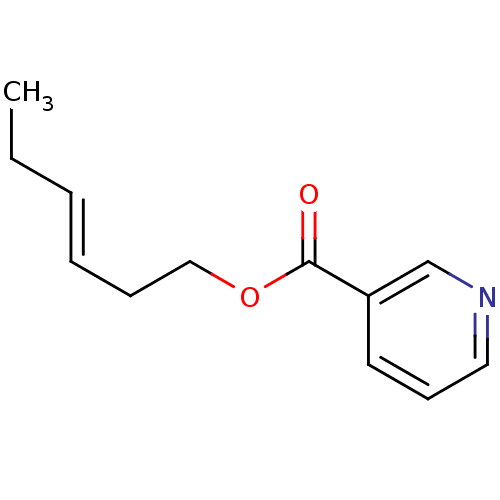 (Auxiliary substrate, 16a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
McGill University | Assay Description All UV/Vis spectra of the enzyme-ligand complexes were obtained on a Cary 5000 UV/Vis spectrophotometer. | Chembiochem 13: 2527-36 (2012) Article DOI: 10.1002/cbic.201200524 BindingDB Entry DOI: 10.7270/Q2PR7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |