

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50177318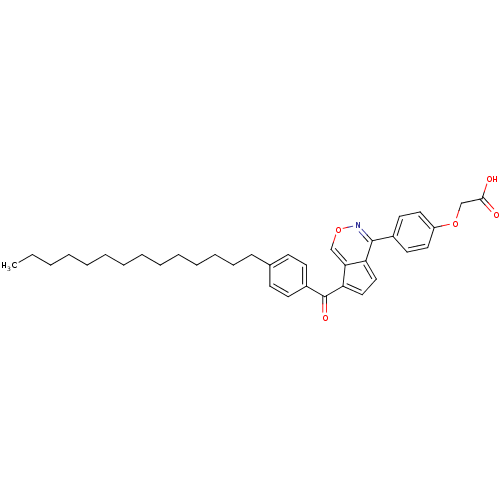 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against cdc25B phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against CD45 phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against Protein phosphatase 1 | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115725 (3-(1-Indol-1-yl-3,4-dioxo-3,4-dihydro-naphthalen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177324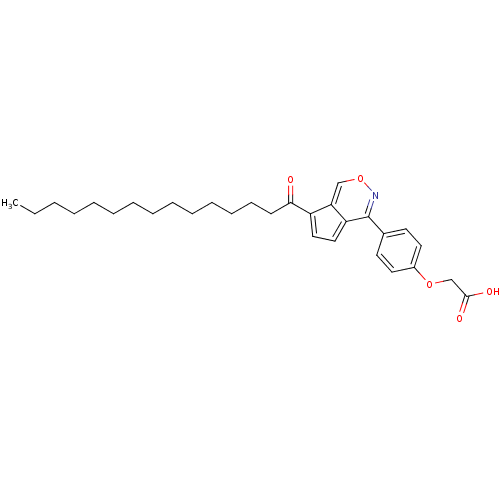 (2-(4-(7-pentadecanoylcyclopenta[d][1,2]oxazin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115722 (4-Cyclohexyl-[1,2]naphthoquinone | CHEMBL58737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115756 (3-(5,6-Dioxo-8-phenyl-5,6-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115733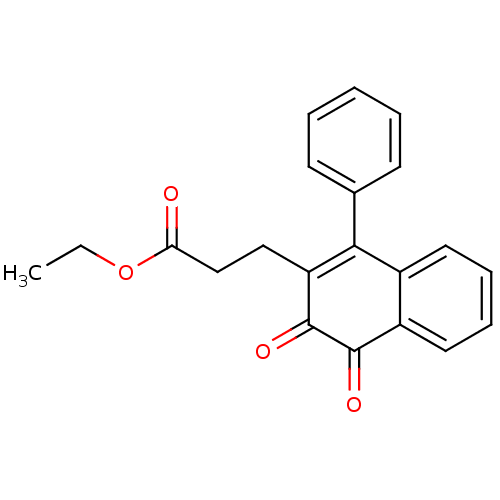 (3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115766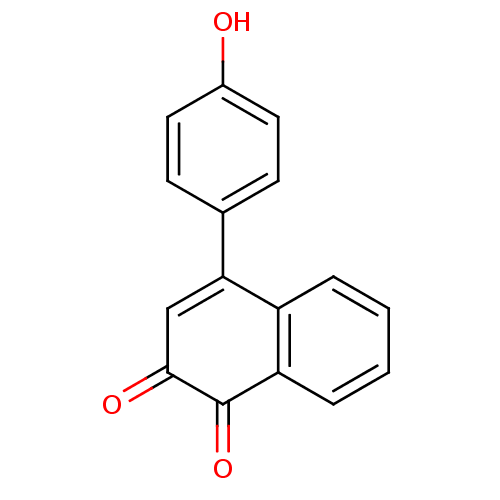 (4-(4-Hydroxy-phenyl)-[1,2]naphthoquinone | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115760 (4-(2,5-Difluoro-phenyl)-[1,2]naphthoquinone | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase YopH (Yersinia enterocolitica) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against YOP protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115721 (3-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115739 (CHEMBL56948 | [4-(1-Indol-1-yl-3,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115740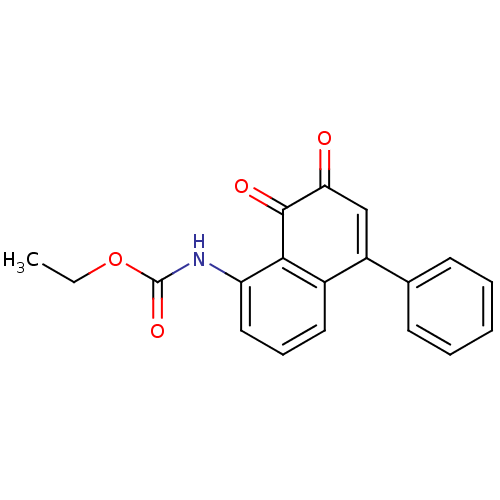 ((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-1-yl)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115746 (3-[7-(2-Methoxycarbonyl-ethyl)-3,4-dioxo-1-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against LAR protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177317 (2-(4-(7-(4-methoxybenzoyl)cyclopenta[d][1,2]oxazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099778 (4-Phenyl-[1,2]naphthoquinone | CHEMBL51447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant human CD45 using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50099778 (4-Phenyl-[1,2]naphthoquinone | CHEMBL51447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115755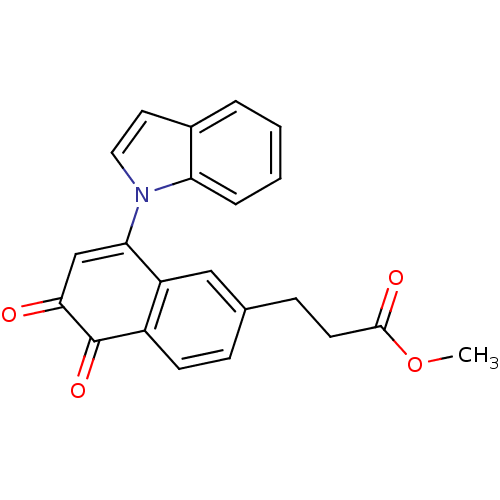 (3-(8-Indol-1-yl-5,6-dioxo-5,6-dihydro-naphthalen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115763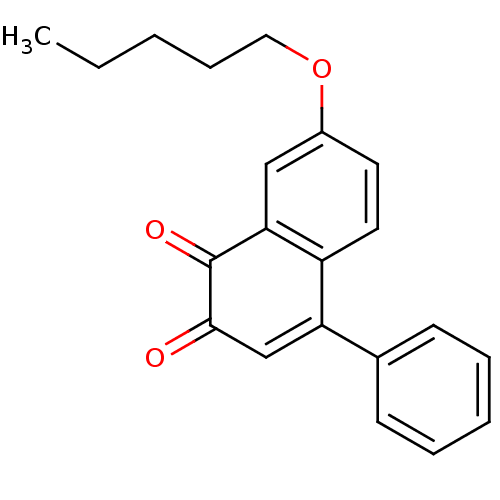 (7-Pentyloxy-4-phenyl-[1,2]naphthoquinone | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115751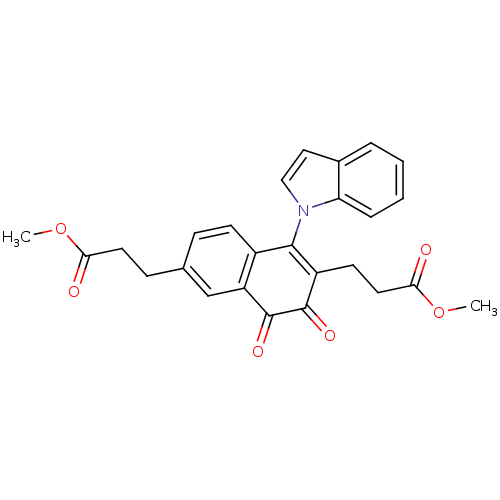 (3-[1-Indol-1-yl-6-(2-methoxycarbonyl-ethyl)-3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115761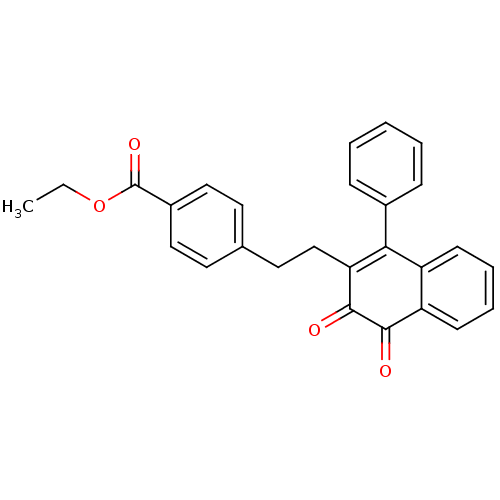 (4-[2-(3,4-Dioxo-1-phenyl-3,4-dihydro-naphthalen-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115728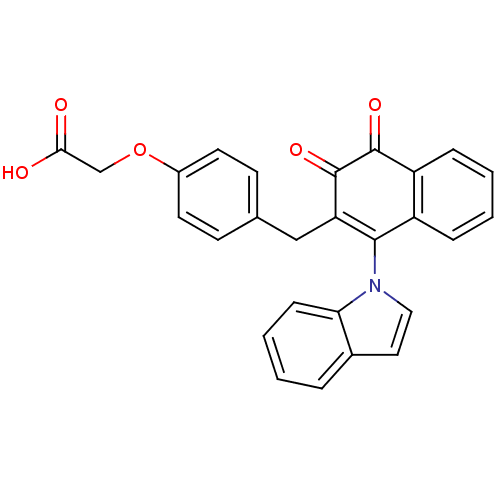 (CHEMBL57841 | [4-(1-Indol-1-yl-3,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115729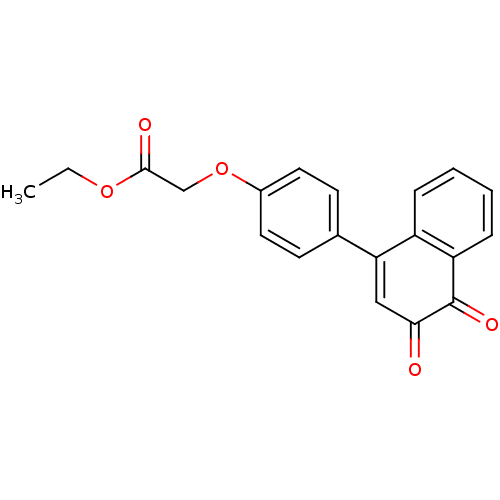 (CHEMBL292843 | [4-(3,4-Dioxo-3,4-dihydro-naphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115737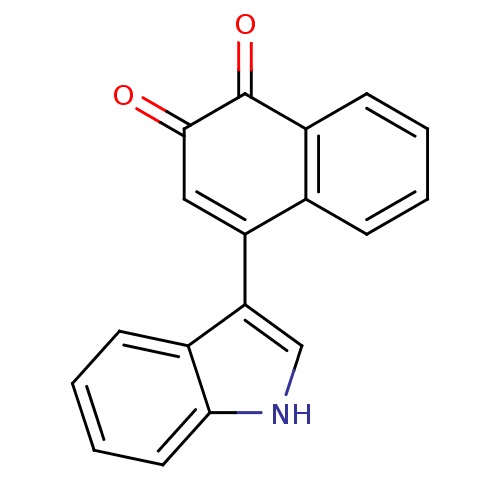 (4-(1H-Indol-3-yl)-[1,2]naphthoquinone | CHEMBL6070...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115727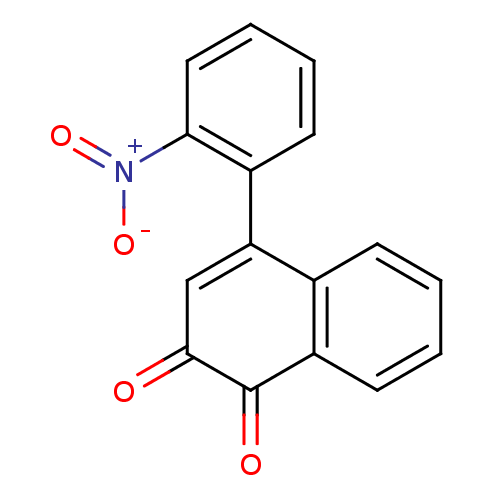 (4-(2-Nitro-phenyl)-[1,2]naphthoquinone | CHEMBL592...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase YopH (Yersinia enterocolitica) | BDBM50177316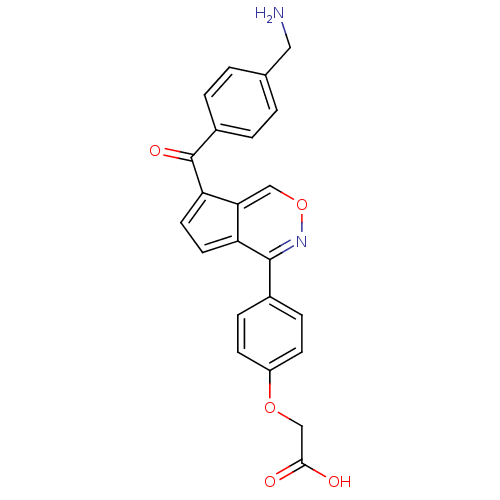 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against YOP protein tyrosine phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50177316 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against CD45 phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (Homo sapiens (Human)) | BDBM50177316 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against Protein phosphatase 1 | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50177318 (2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against cdc25A phosphatase | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115744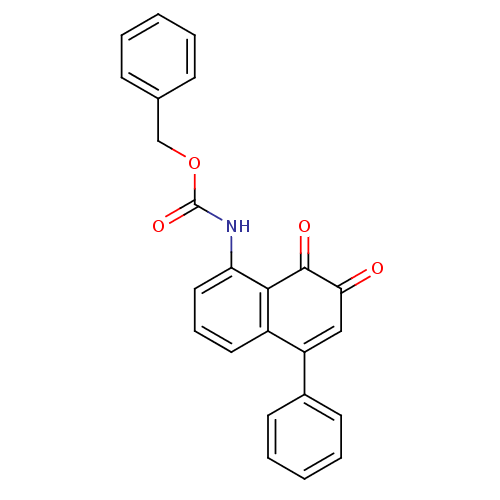 ((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-1-yl)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115765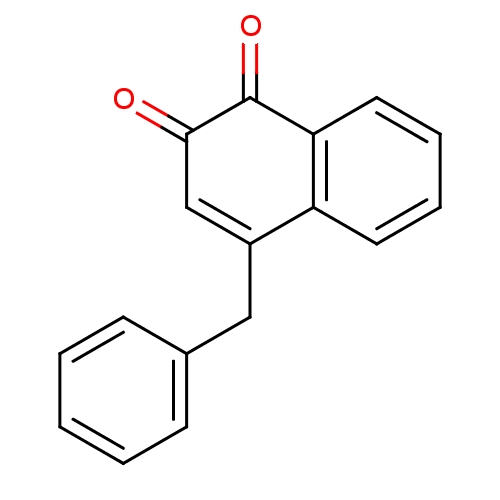 (4-Benzyl-[1,2]naphthoquinone | CHEMBL292386) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115735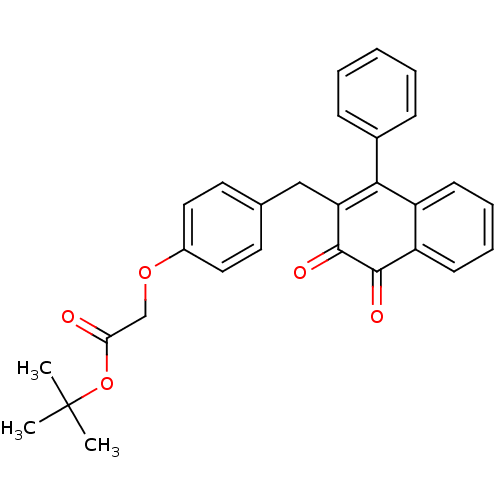 (CHEMBL58354 | [4-(3,4-Dioxo-1-phenyl-3,4-dihydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115748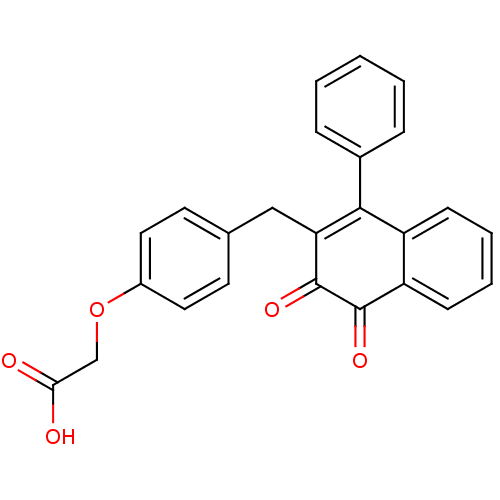 (CHEMBL301436 | [4-(3,4-Dioxo-1-phenyl-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115731 (4-(3,4-Dioxo-3,4-dihydro-naphthalen-1-yl)-benzoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115738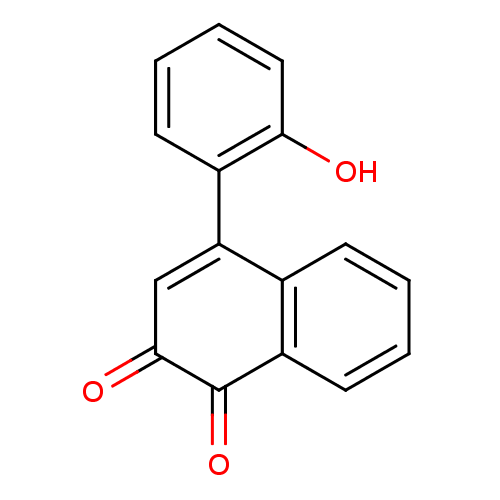 (4-(2-Hydroxy-phenyl)-[1,2]naphthoquinone | CHEMBL6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM22851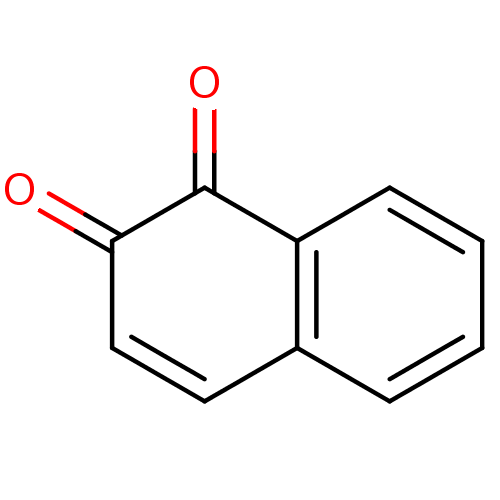 (1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177322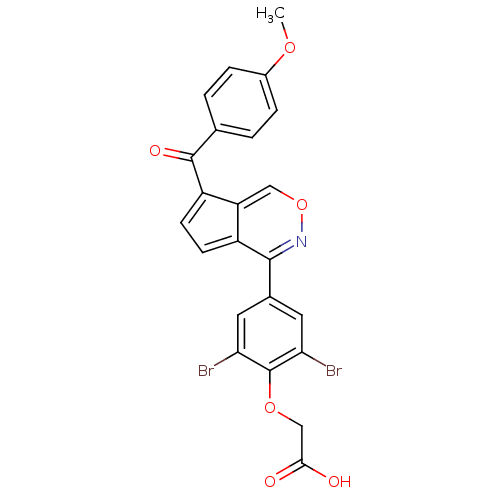 (2-(2,6-dibromo-4-(7-(4-methoxybenzoyl)cyclopenta[d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50115731 (4-(3,4-Dioxo-3,4-dihydro-naphthalen-1-yl)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant human CD45 using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50115737 (4-(1H-Indol-3-yl)-[1,2]naphthoquinone | CHEMBL6070...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant human Cdc25B using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177320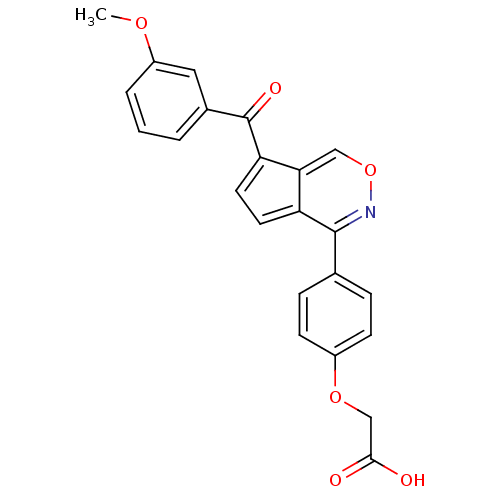 (2-(4-(7-(3-methoxybenzoyl)cyclopenta[d][1,2]oxazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115743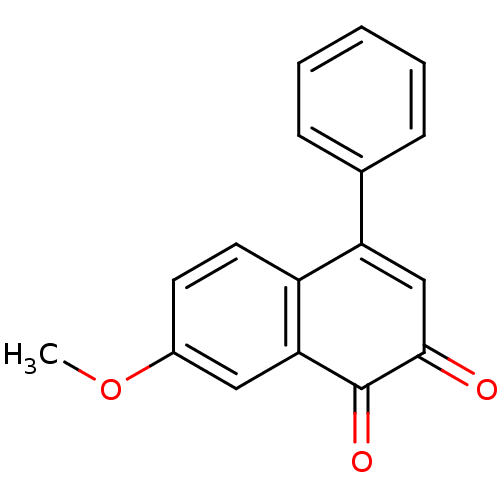 (7-Methoxy-4-phenyl-[1,2]naphthoquinone | CHEMBL587...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115764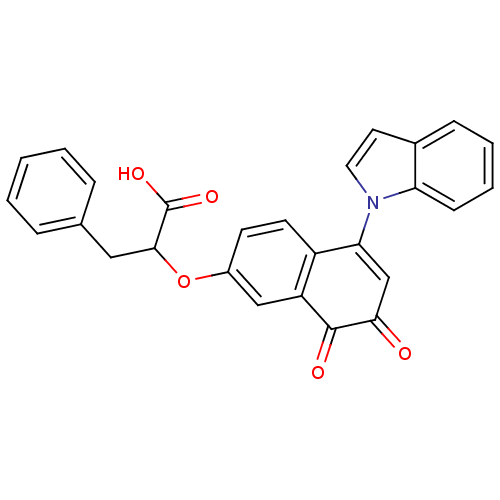 (2-(5-Indol-1-yl-7,8-dioxo-7,8-dihydro-naphthalen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50115731 (4-(3,4-Dioxo-3,4-dihydro-naphthalen-1-yl)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant human Cdc25B using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115749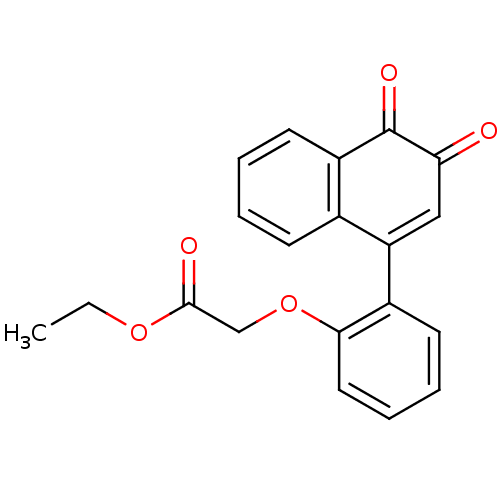 (CHEMBL57036 | [2-(3,4-Dioxo-3,4-dihydro-naphthalen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115758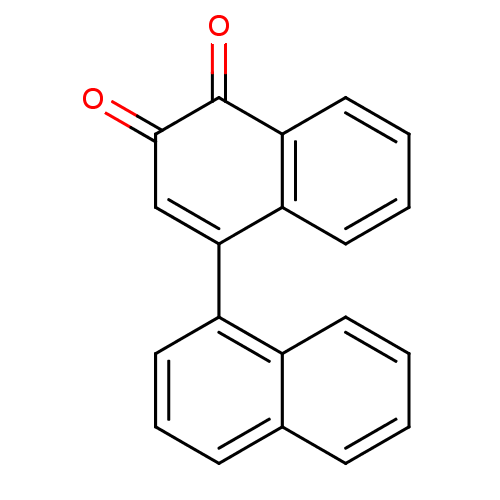 (CHEMBL59240 | [1,1']Binaphthalenyl-3,4-dione) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50177316 (2-(4-(7-(4-(aminomethyl)benzoyl)cyclopenta[d][1,2]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human PTP1B | Bioorg Med Chem Lett 16: 499-502 (2005) Article DOI: 10.1016/j.bmcl.2005.10.062 BindingDB Entry DOI: 10.7270/Q2319VFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50115757 ((7,8-Dioxo-5-phenyl-7,8-dihydro-naphthalen-2-yl)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human protein-tyrosine phosphatase 1B (PTP1B) using fluoreacein diphosphate (FDP) as a substrate | Bioorg Med Chem Lett 12: 1941-6 (2002) BindingDB Entry DOI: 10.7270/Q2QZ2999 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |