

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50090249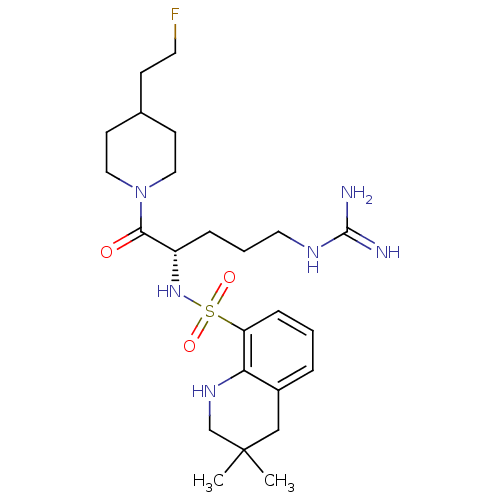 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213911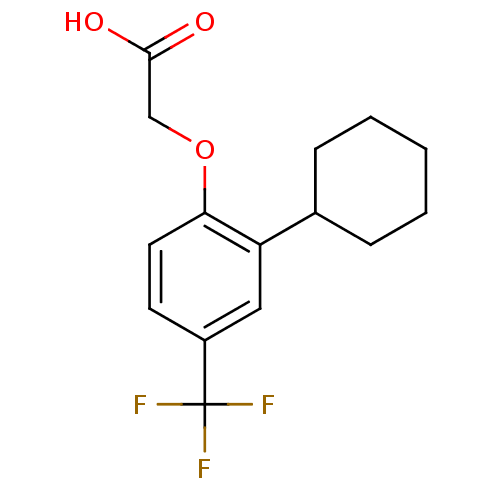 (2-(2-cyclohexyl-4-(trifluoromethyl)phenoxy)acetic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213919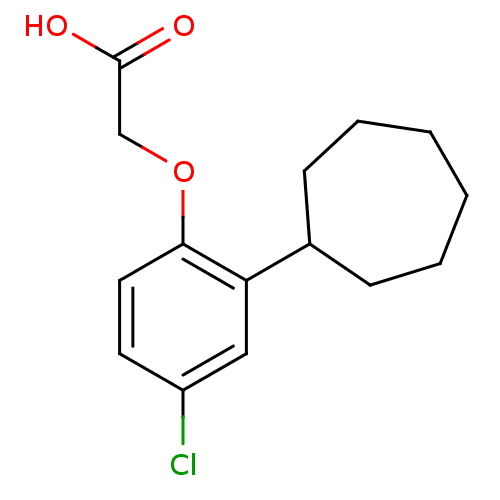 (2-(4-chloro-2-cycloheptylphenoxy)acetic acid | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213912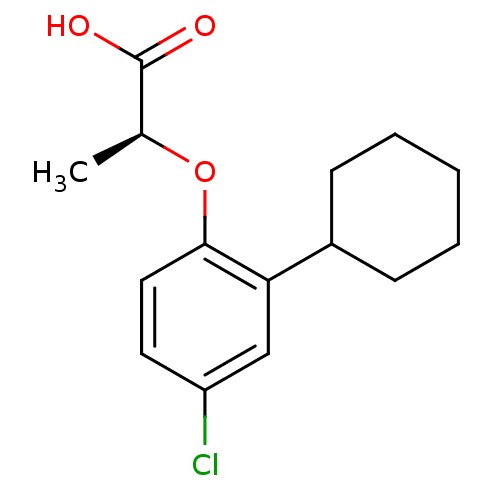 ((S)-2-(4-chloro-2-cyclohexylphenoxy)propanoic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213922 (2-(4-bromo-2-cyclohexylphenoxy)acetic acid | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213908 (2-(4-chloro-2-(1-methylcyclohexyl)phenoxy)acetic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213907 ((3-cyclohexyl-4'-fluoro-biphenyl-4-yloxy)-acetic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226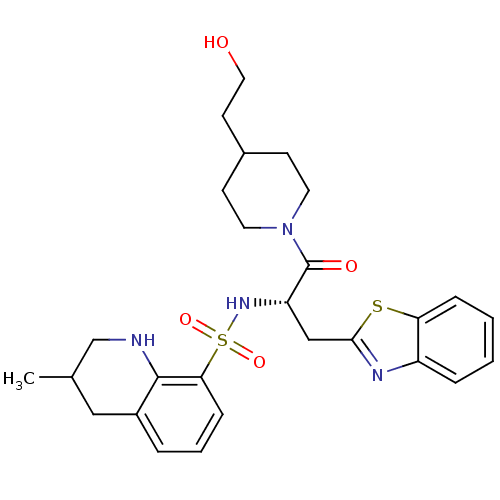 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366643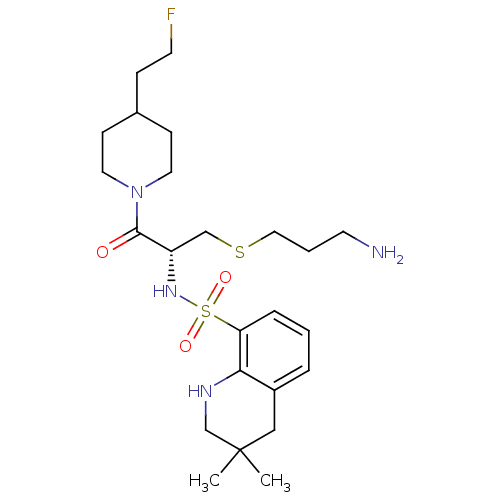 (CHEMBL1907778) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213917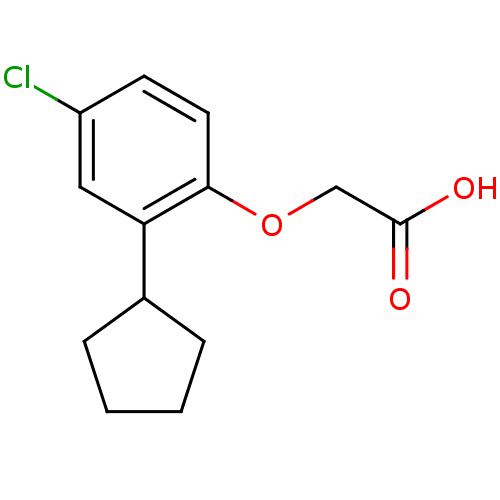 (2-(4-chloro-2-cyclopentylphenoxy)acetic acid | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213909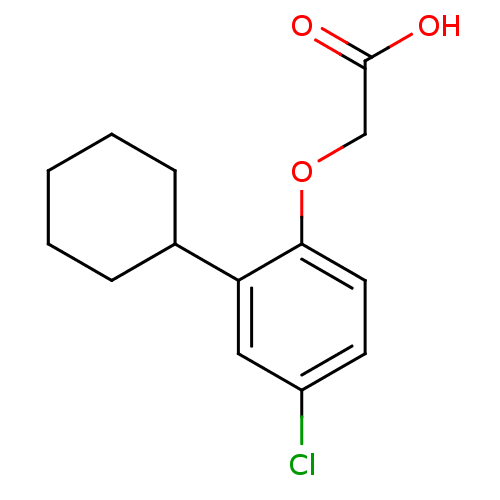 (2-(4-chloro-2-cyclohexylphenoxy)acetic acid | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213915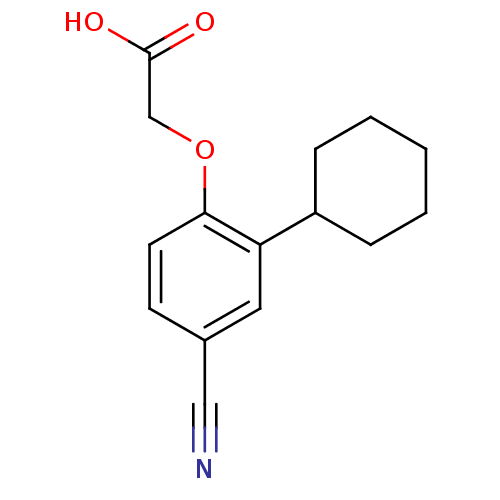 (2-(4-cyano-2-cyclohexylphenoxy)acetic acid | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090231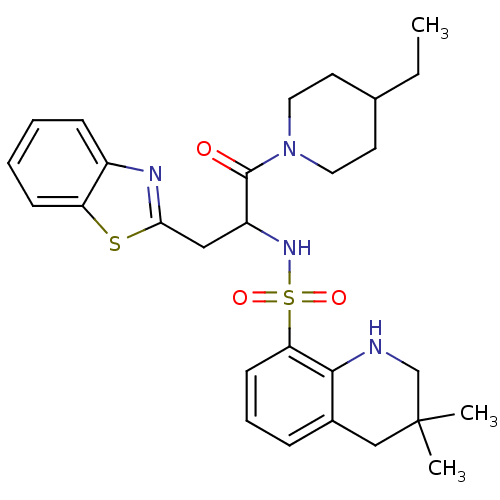 (CHEMBL39375 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366642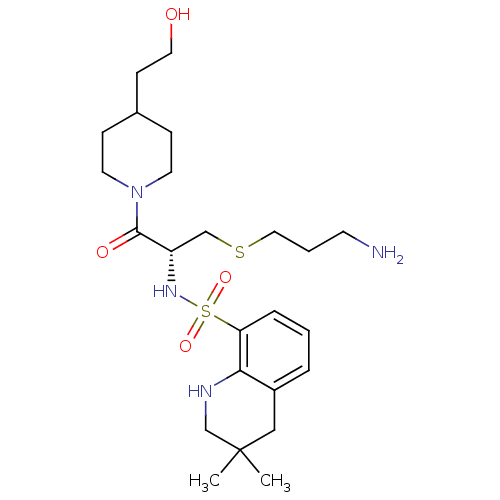 (CHEMBL1907779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213918 (2-(2-cyclohexylphenoxy)acetic acid | CHEMBL247739) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213926 (2-(2-cyclohexyl-4-methylphenoxy)acetic acid | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213913 (2-(2-cyclohexyl-4-fluorophenoxy)acetic acid | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 446 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090236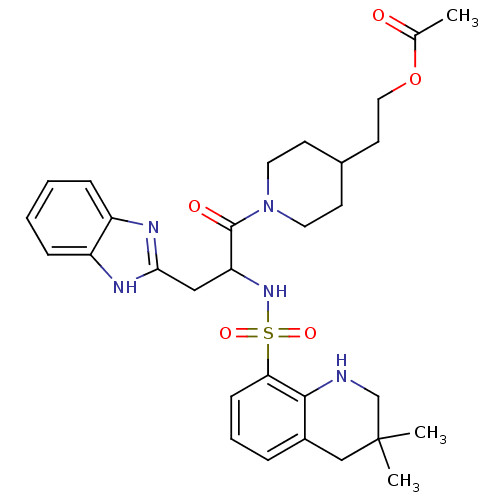 (CHEMBL418248 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090235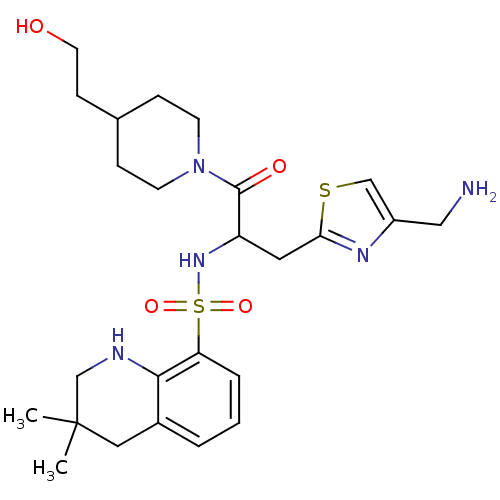 (CHEMBL38907 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090237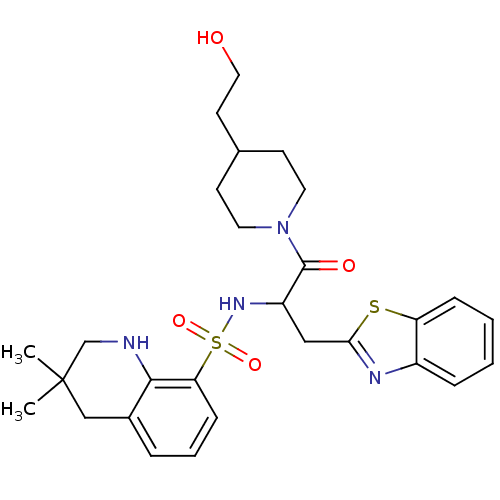 (CHEMBL43074 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213921 (2-(2-cyclohexyl-4-methoxyphenoxy)acetic acid | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 782 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213910 (3-(4-chloro-2-cyclohexylphenoxy)propanoic acid | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 799 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090220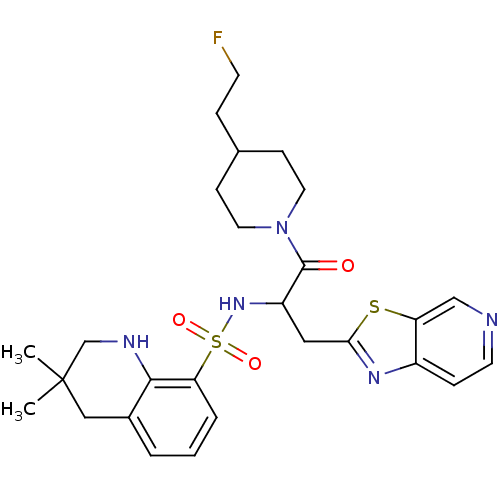 (CHEMBL289623 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213908 (2-(4-chloro-2-(1-methylcyclohexyl)phenoxy)acetic a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090234 (CHEMBL40904 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213919 (2-(4-chloro-2-cycloheptylphenoxy)acetic acid | CHE...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213928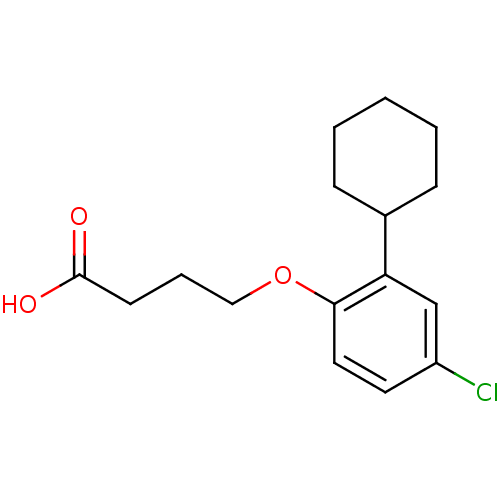 (4-(4-chloro-2-cyclohexylphenoxy)butanoic acid | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090230 (CHEMBL38617 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213920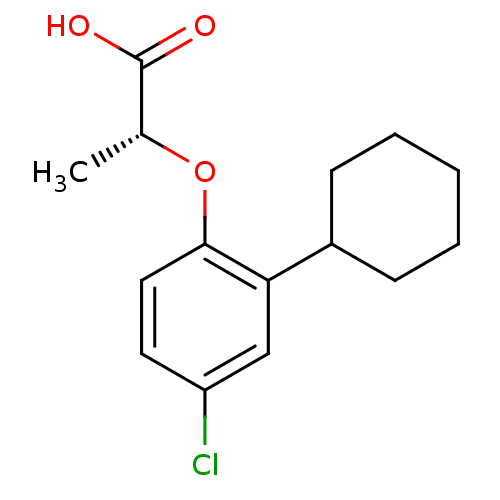 ((R)-2-(4-chloro-2-cyclohexylphenoxy)propanoic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50213909 (2-(4-chloro-2-cyclohexylphenoxy)acetic acid | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid EP2 receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090224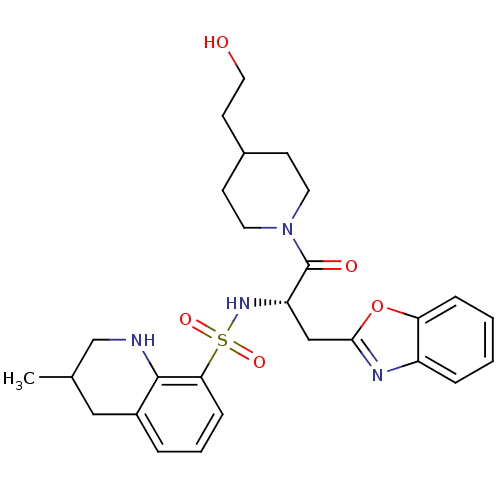 (CHEMBL289493 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090227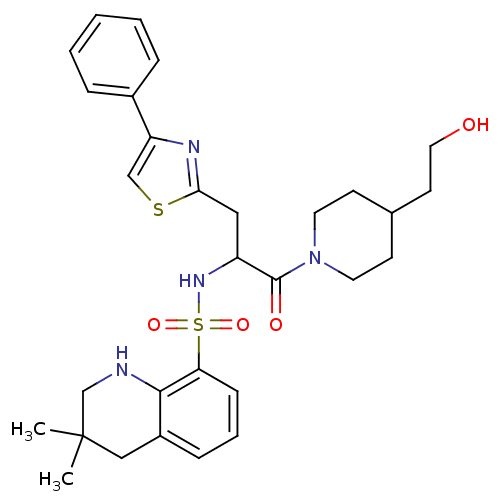 (CHEMBL288938 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213924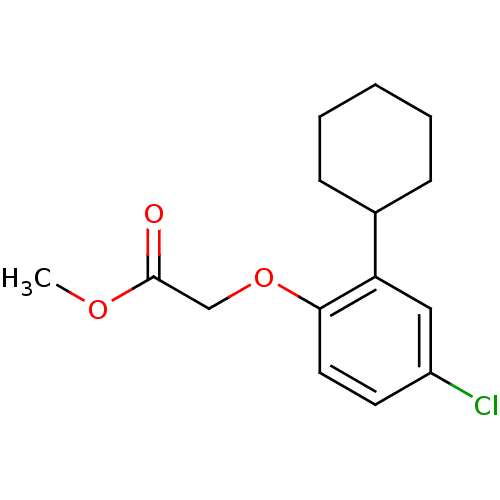 (CHEMBL247131 | methyl 2-(4-chloro-2-cyclohexylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213912 ((S)-2-(4-chloro-2-cyclohexylphenoxy)propanoic acid...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090228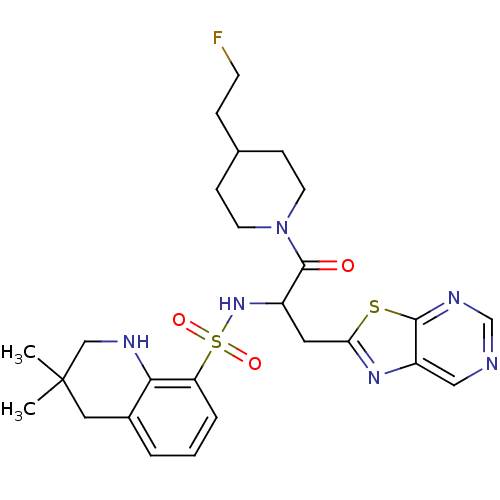 (CHEMBL289628 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213925 (2-(2-allyl-4-chlorophenoxy)acetic acid | CHEMBL245...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213917 (2-(4-chloro-2-cyclopentylphenoxy)acetic acid | CHE...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090231 (CHEMBL39375 | MD805 Analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50213925 (2-(2-allyl-4-chlorophenoxy)acetic acid | CHEMBL245...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213922 (2-(4-bromo-2-cyclohexylphenoxy)acetic acid | CHEMB...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50213907 ((3-cyclohexyl-4'-fluoro-biphenyl-4-yloxy)-acetic a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Binding affinity at prostanoid DP receptor | Bioorg Med Chem Lett 17: 4347-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.019 BindingDB Entry DOI: 10.7270/Q2N58M2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090225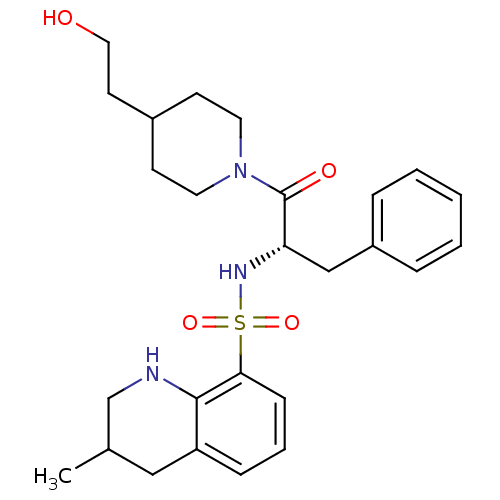 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 672 total ) | Next | Last >> |