

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041043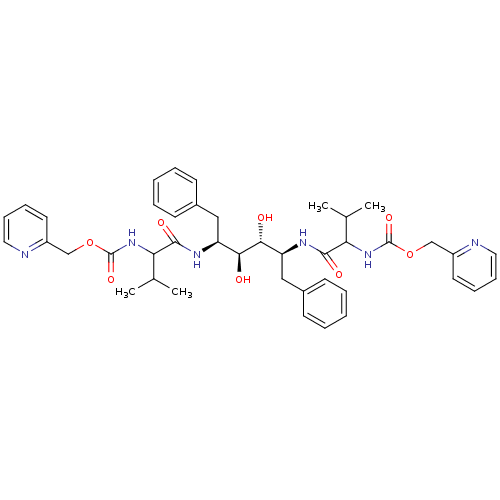 ((1-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041045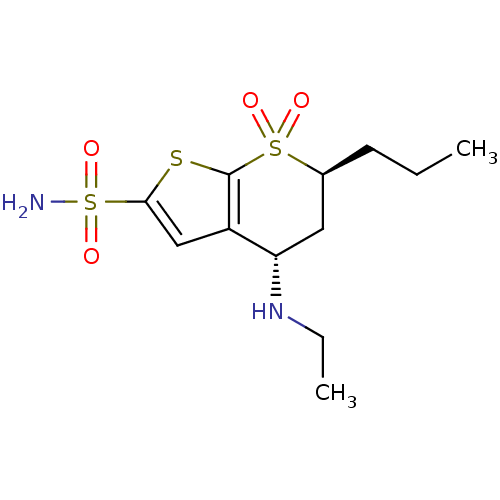 ((4S,6S)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197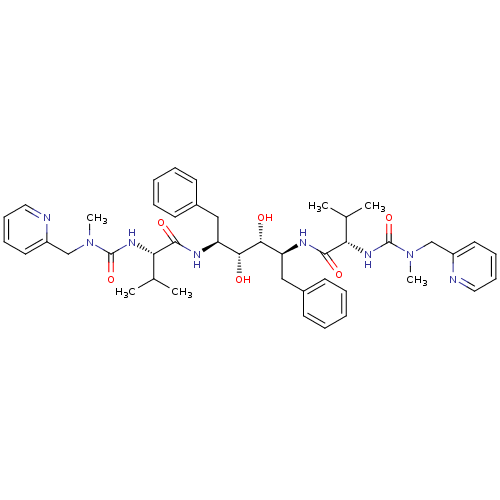 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041023 ((1-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041037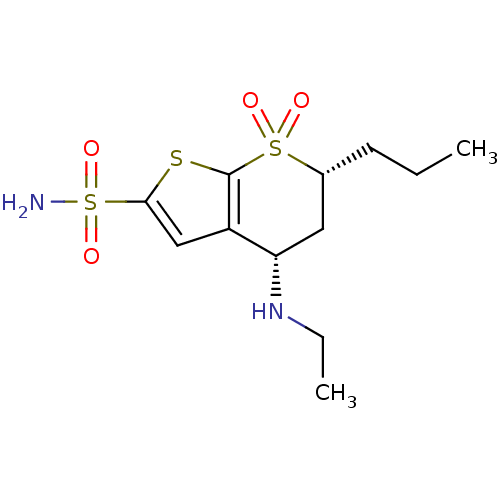 ((4S,6R)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041035 (CHEMBL269401 | N-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041032 ((1-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50318494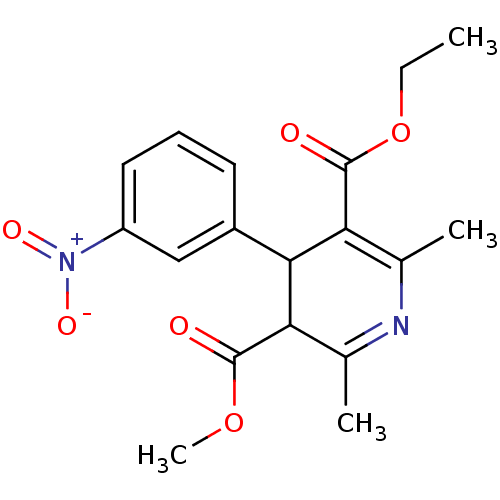 (3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesicles | J Med Chem 30: 690-5 (1987) BindingDB Entry DOI: 10.7270/Q2BC404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041027 ((4S,6S)-6-Ethyl-4-ethylamino-7,7-dioxo-4,5,6,7-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029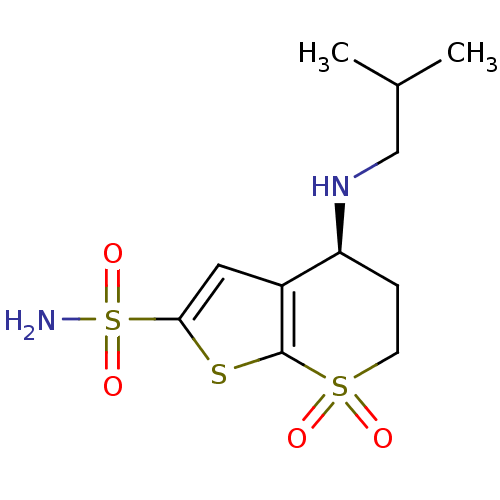 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017729 (4-Ethylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50021195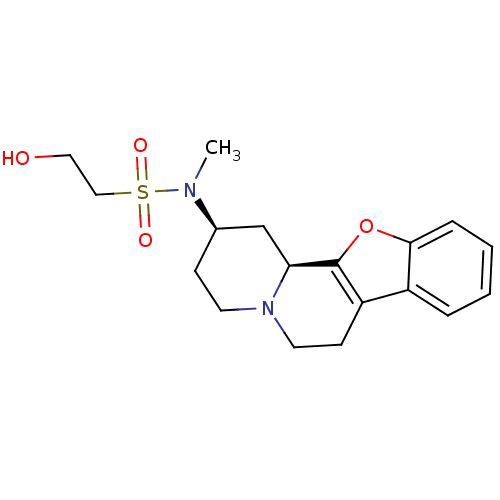 (2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortex | J Med Chem 28: 1756-9 (1986) BindingDB Entry DOI: 10.7270/Q2JW8CWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50367851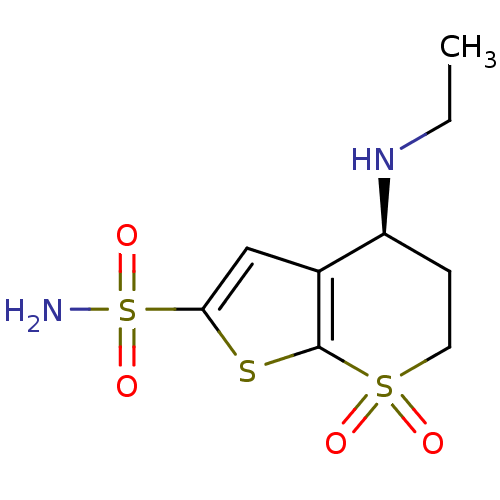 (CHEMBL1788291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation at 3 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50016400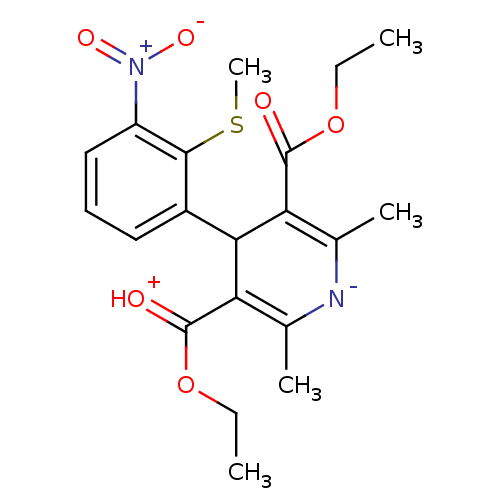 (2,6-Dimethyl-4-(2-methylsulfanyl-3-nitro-phenyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesicles | J Med Chem 30: 690-5 (1987) BindingDB Entry DOI: 10.7270/Q2BC404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041048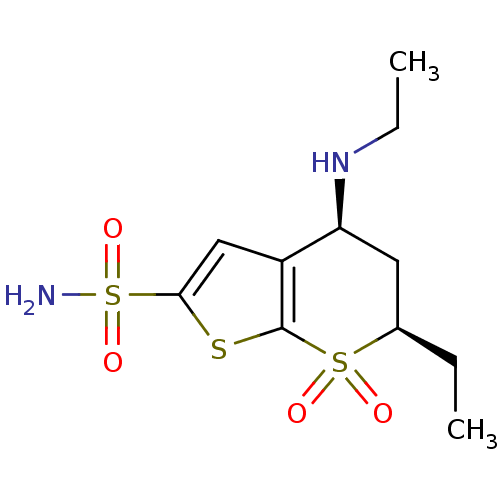 ((4S,6R)-6-Ethyl-4-ethylamino-7,7-dioxo-4,5,6,7-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesicles | J Med Chem 30: 690-5 (1987) BindingDB Entry DOI: 10.7270/Q2BC404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50021195 (2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of human plasma renin | J Med Chem 28: 1756-9 (1986) BindingDB Entry DOI: 10.7270/Q2JW8CWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017728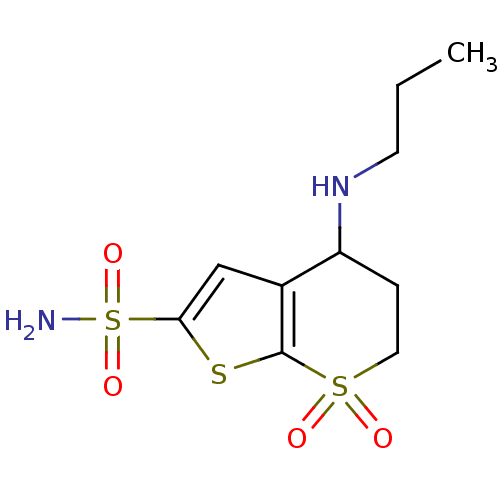 (7,7-Dioxo-4-propylamino-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024220 (7-Oxo-6,7-dihydro-5H-thieno[3,2-b]thiopyran-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50021196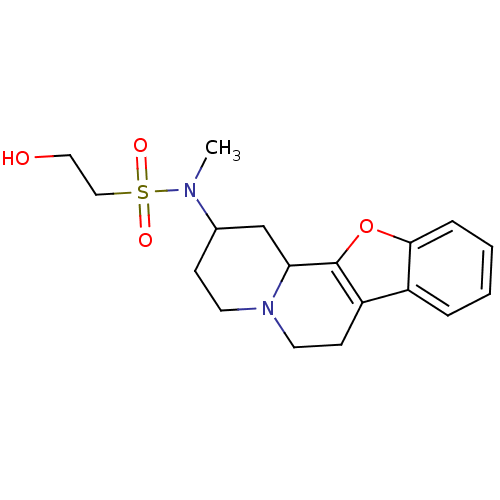 (2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortex | J Med Chem 28: 1756-9 (1986) BindingDB Entry DOI: 10.7270/Q2JW8CWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041033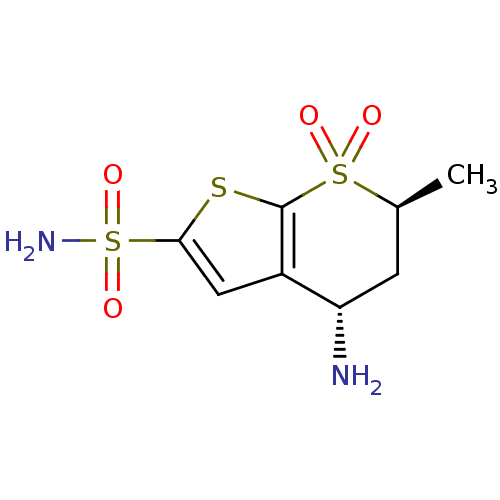 ((4S,6S)-4-Amino-6-methyl-7,7-dioxo-4,5,6,7-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50021196 (2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of hog kidney renin | J Med Chem 28: 1756-9 (1986) BindingDB Entry DOI: 10.7270/Q2JW8CWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041026 (CHEMBL10113 | N-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017726 (4-Butylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041041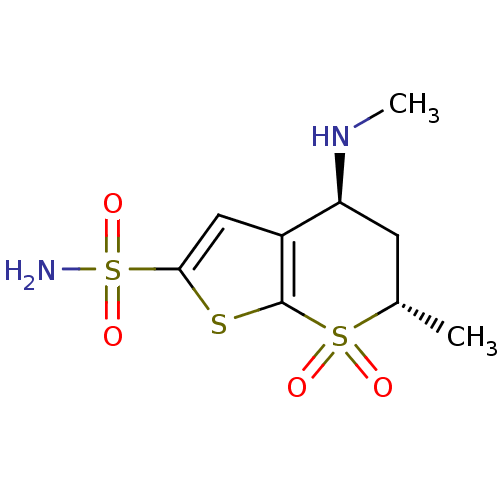 ((4S,6S)-6-Methyl-4-methylamino-7,7-dioxo-4,5,6,7-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005210 ((2-Imino-1-methyl-1,2-dihydro-benzo[cd]indol-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005186 ((1,5-Dimethyl-2-methylimino-1,2-dihydro-benzo[cd]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50227000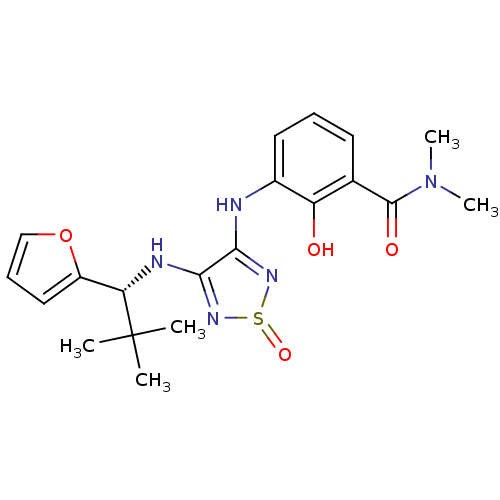 (3-[4-((R)-1-furan-2-yl-2,2-dimethyl-propylamino)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017732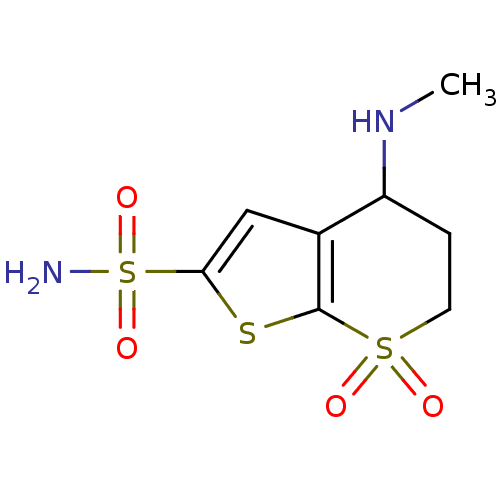 (4-Methylamino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50227005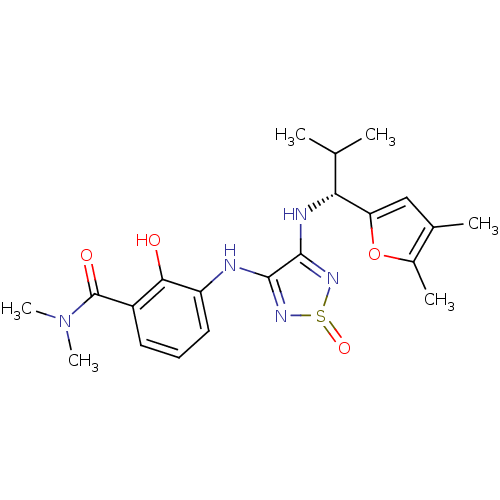 (3-{4-[(R)-1-(4,5-dimethyl-furan-2-yl)-2-methyl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50226999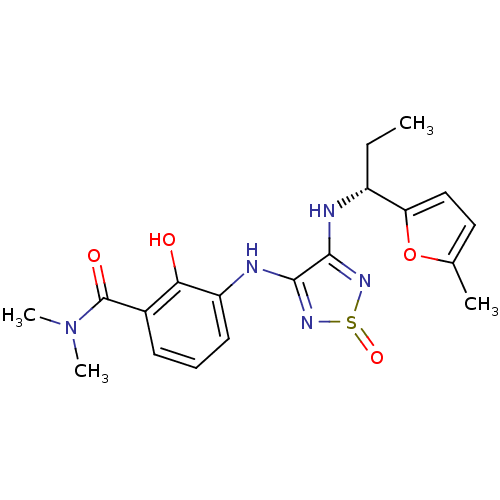 (2-hydroxy-N,N-dimethyl-3-{4-[(R)-1-(5-methyl-furan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024224 (7,7-Dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding to human erythrocyte carbonic anhydrase was determined by fluorescence competition assay employing the fluorescent CA inhibitor dans... | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50024221 (4-Oxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017731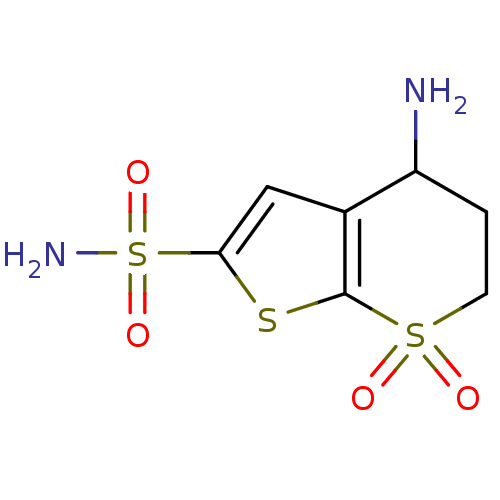 (4-Amino-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description The equilibrium dissociation constant of the inhibitor-enzyme complex of human Carbonic anhydrase II | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50226995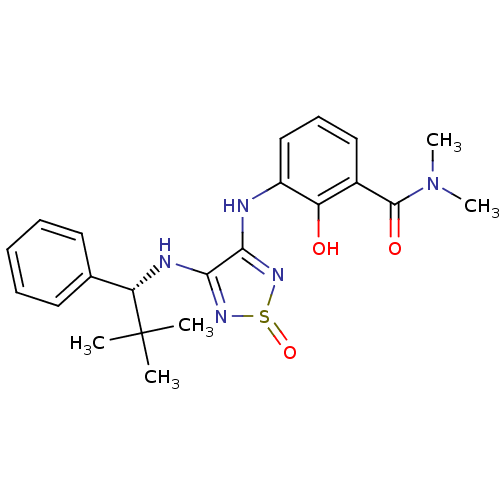 (3-[4-((R)-2,2-dimethyl-1-phenyl-propylamino)-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404821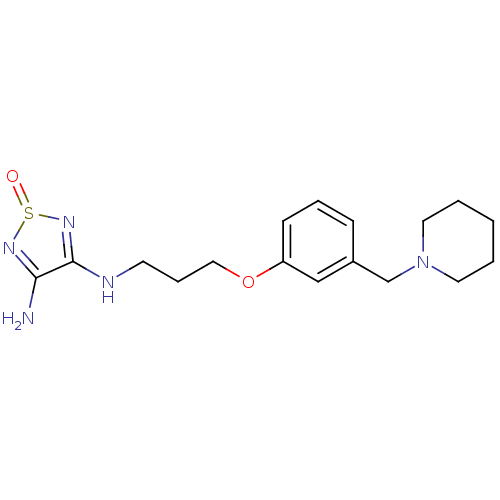 (CHEMBL306465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018739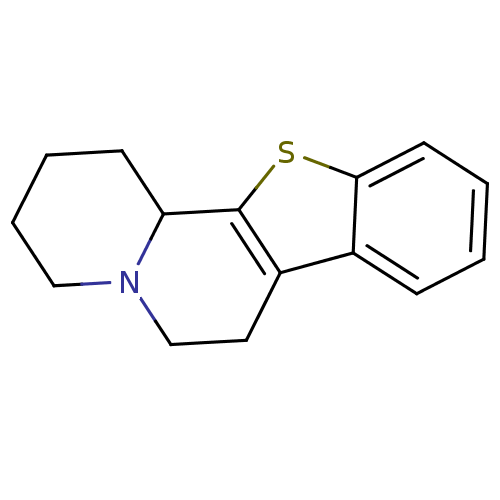 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (BOVINE) | BDBM50018739 (1,3,4,5,6,11b-Hexahydro-2H-11-thia-4a-aza-benzo[a]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Affinity to alpha-2 adrenergic receptor by the displacement of [3H]-clonidine from calf cerebral cortex membranes | J Med Chem 31: 641-5 (1988) BindingDB Entry DOI: 10.7270/Q208649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50227003 (3-[4-((R)-1-benzo[1,3]dioxol-5-yl-2,2-dimethyl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50227001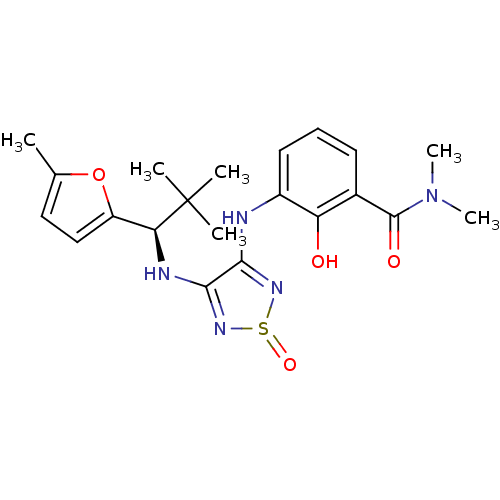 (3-{4-[(R)-2,2-dimethyl-1-(5-methyl-furan-2-yl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50226996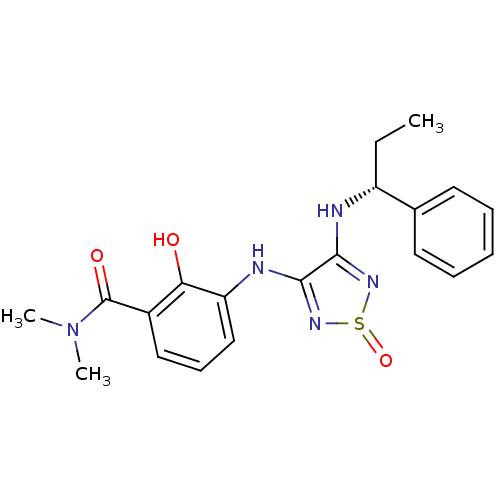 (2-hydroxy-N,N-dimethyl-3-[1-oxo-4-((R)-1-phenyl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | Bioorg Med Chem Lett 18: 228-31 (2008) Article DOI: 10.1016/j.bmcl.2007.10.094 BindingDB Entry DOI: 10.7270/Q25B027K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50452418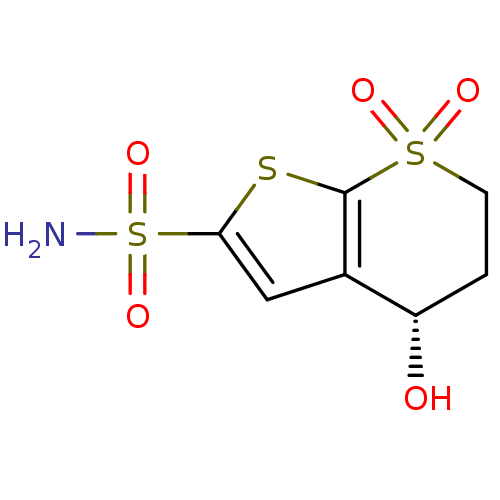 (CHEMBL2092886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme-Chibret Curated by ChEMBL | Assay Description 50% inhibitory concentration against human carbonic anhydrase II (HCA II) after pre-incubation for 4 min at 37 degree C | J Med Chem 32: 2510-3 (1989) BindingDB Entry DOI: 10.7270/Q29W0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404823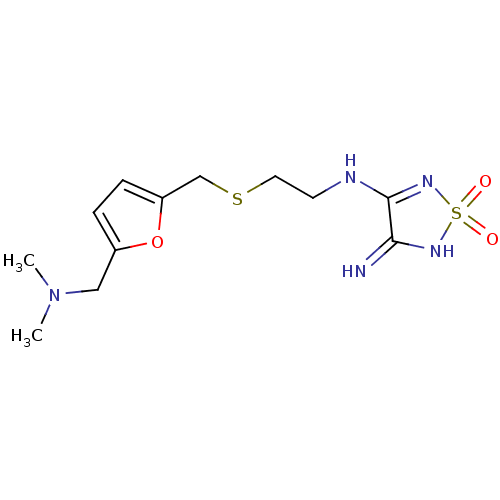 (CHEMBL63299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404822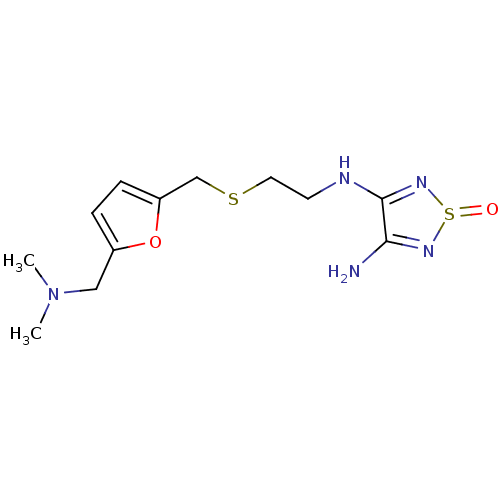 (CHEMBL8982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50452418 (CHEMBL2092886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against purified human erythrocyte carbonic anhydrase II was determined (acetozolamide used as control.) | J Med Chem 30: 591-7 (1987) BindingDB Entry DOI: 10.7270/Q2KW5GMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 801 total ) | Next | Last >> |