

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50022633 (CHEMBL3142281 | [1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022635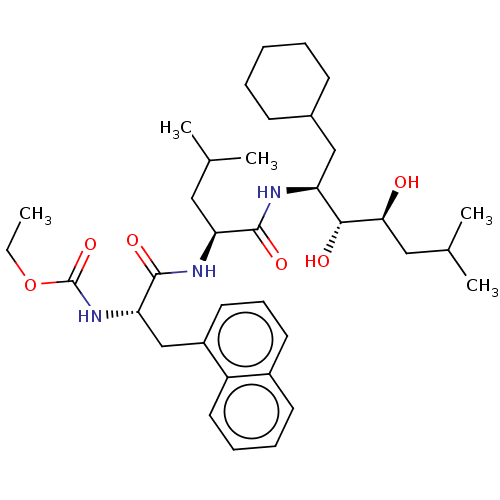 (CHEMBL3142255 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022597 (CHEMBL3142268 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022619 (CHEMBL3348544 | N-[1-(1-Cyclohexylmethyl-2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022603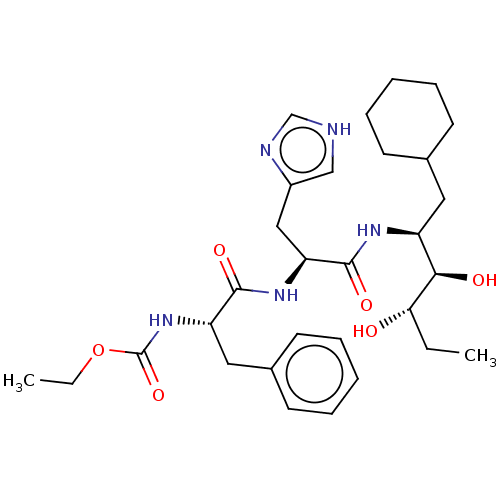 (CHEMBL3348551 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022628 (CHEMBL3142261 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022599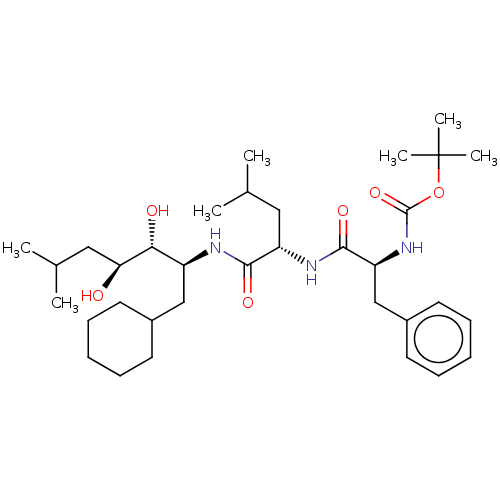 (CHEMBL3142262 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022598 (CHEMBL3348548 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022595 (CHEMBL3348552 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022630 (CHEMBL3348531 | Cyclohexanecarboxylic acid {1-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022637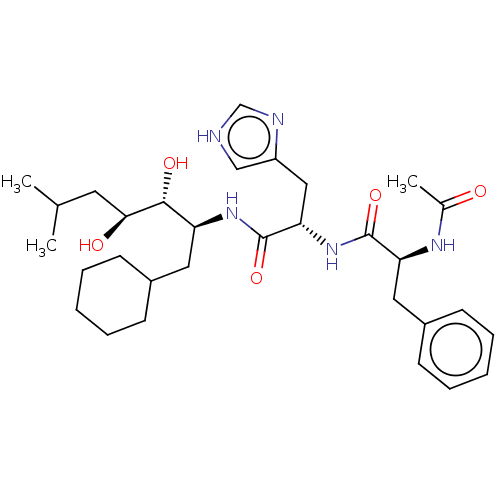 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022593 (CHEMBL3142304 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022620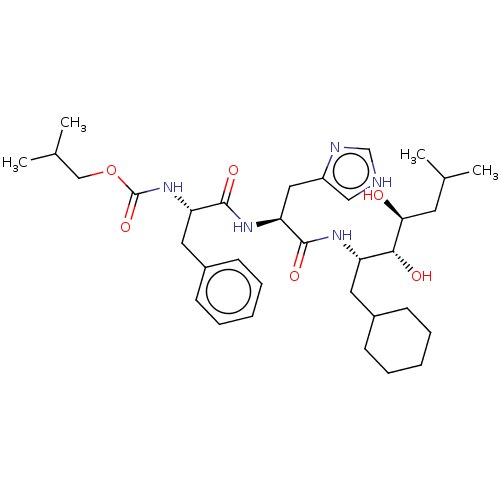 (CHEMBL3348532 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022611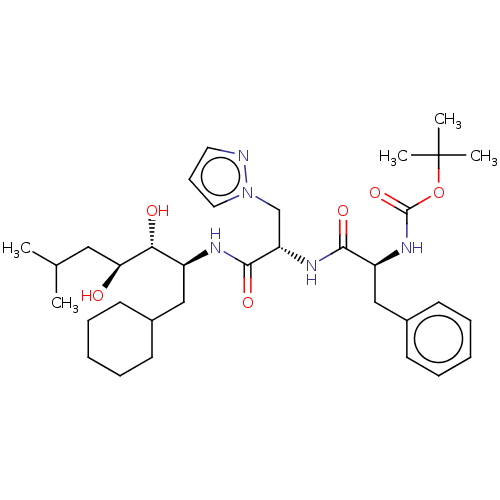 (CHEMBL3142260 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022607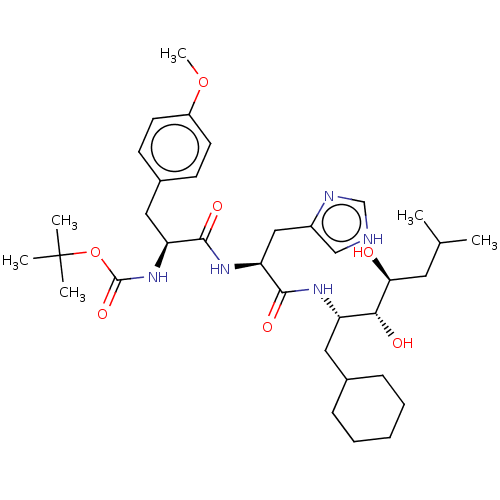 (CHEMBL3348540 | [1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022615 (CHEMBL3348546 | N-{1-[1-(1-Cyclohexylmethyl-2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022638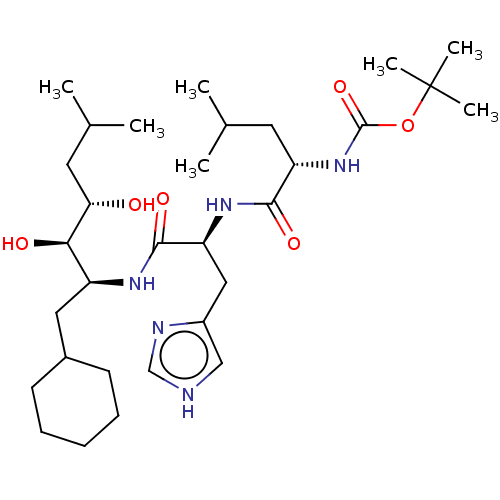 (CHEMBL3348542 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022636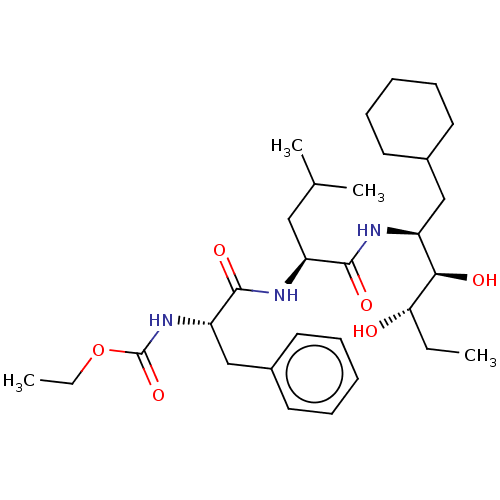 (CHEMBL3142269 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022625 (CHEMBL3142263 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022613 (CHEMBL430933 | {1-[1-(1-Cyclohexylmethyl-2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022610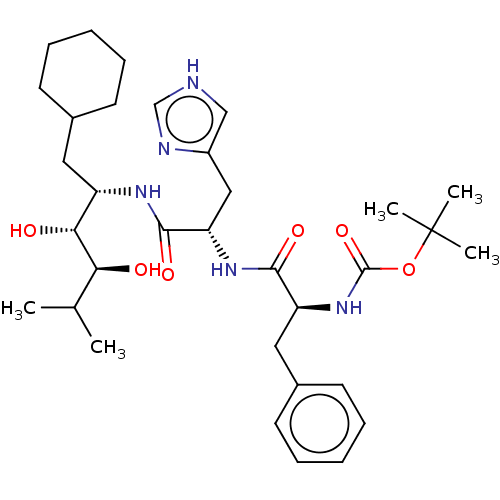 (CHEMBL3348557 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022616 (CHEMBL3348555 | [1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022634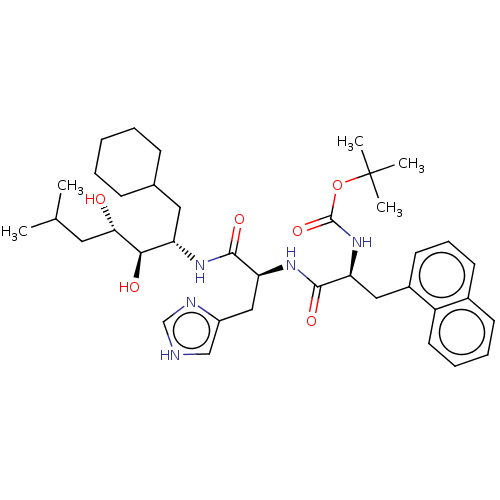 (CHEMBL3348541 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50068561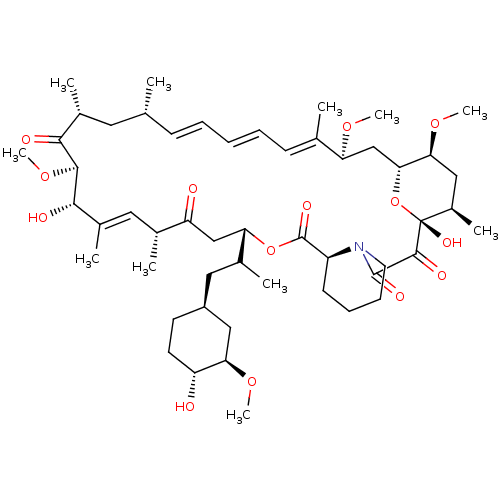 ((16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022629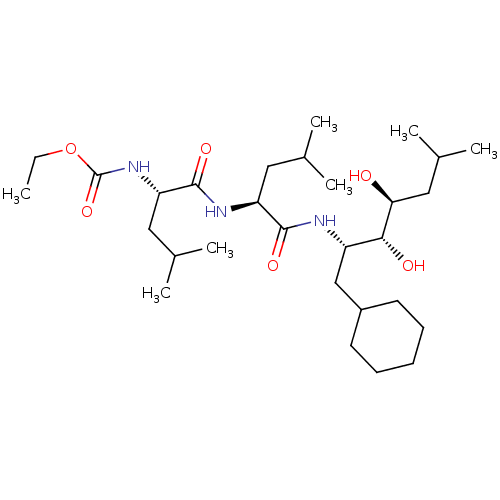 (CHEMBL3142270 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022600 (CHEMBL3348538 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022631 (2-(3-tert-Butyl-ureido)-N-[1-(1-cyclohexylmethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022624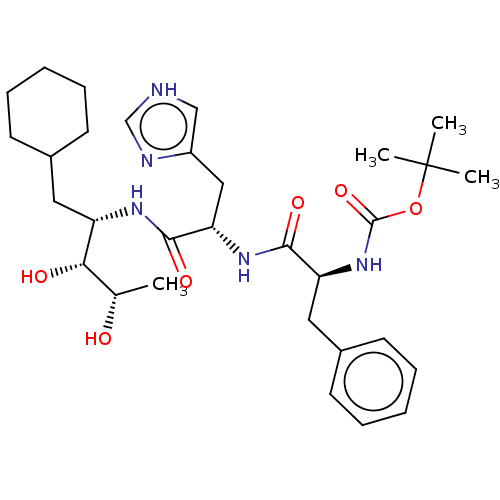 (CHEMBL3348549 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006132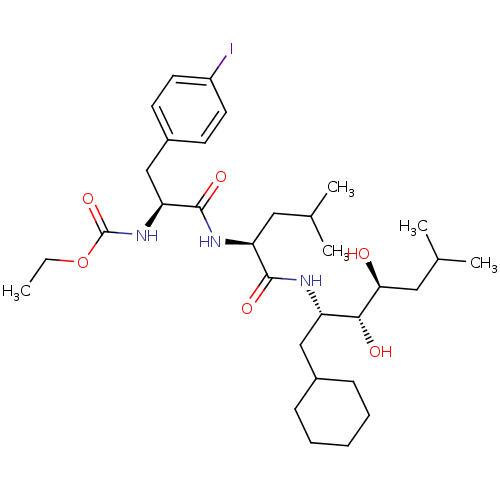 (CHEMBL297929 | [1-[1-(1-Cyclohexylmethyl-2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022617 (CHEMBL3142274 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [671-1210,L858R] (Homo sapiens (Human)) | BDBM27970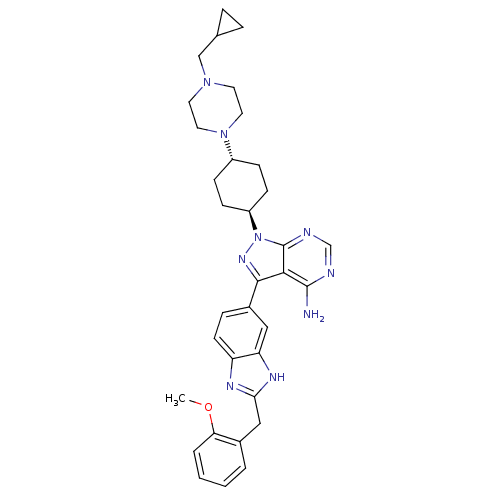 (1-{4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohex...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... | Bioorg Med Chem Lett 19: 1718-21 (2009) Article DOI: 10.1016/j.bmcl.2009.01.086 BindingDB Entry DOI: 10.7270/Q2CJ8BTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174276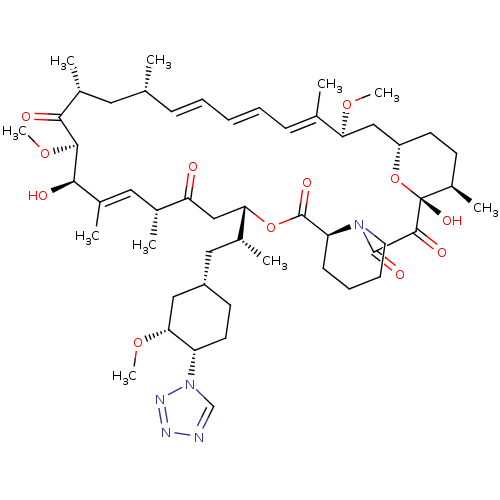 ((1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022883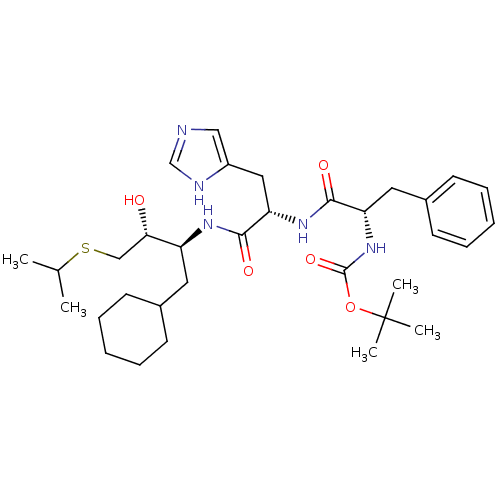 (1N-[1-[1-cyclohexylmethyl-2-hydroxy-3-isopropylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human renal renin at pH 6 | J Med Chem 31: 532-9 (1988) BindingDB Entry DOI: 10.7270/Q2NK3D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022640 (CHEMBL3348556 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50315895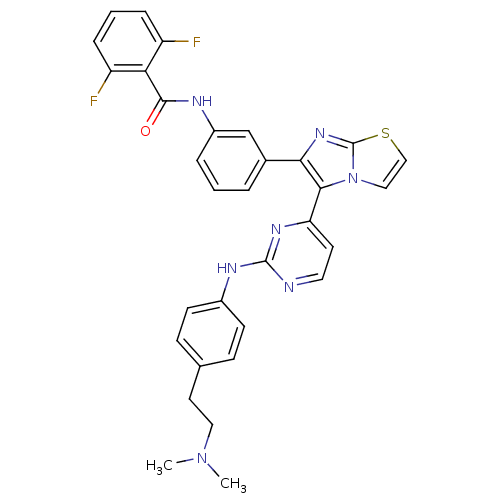 (CHEMBL1090357 | N-(3-(5-(2-(4-(2-(dimethylamino)et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of ErbB2 by time resolved fluorescence assay | Bioorg Med Chem Lett 20: 2452-5 (2010) Article DOI: 10.1016/j.bmcl.2010.03.015 BindingDB Entry DOI: 10.7270/Q2M908VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174275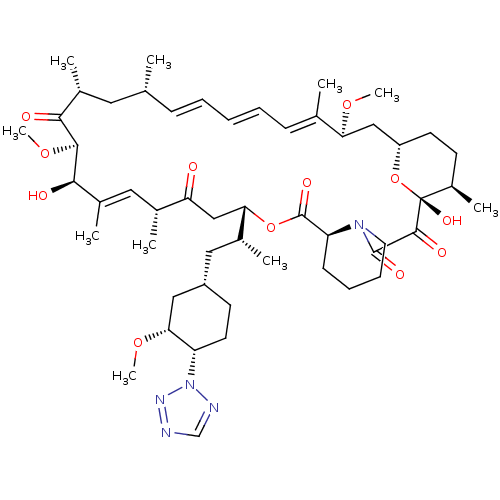 ((1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50315905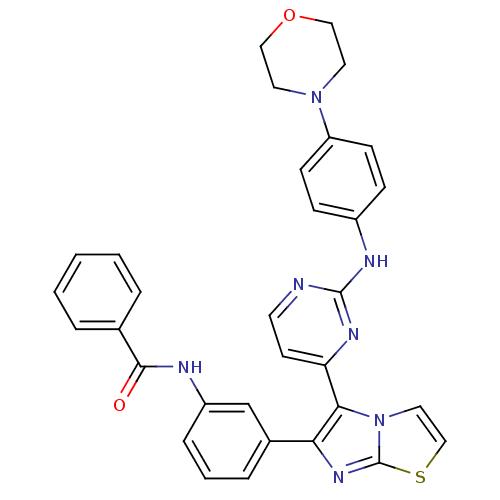 (CHEMBL1090350 | N-(3-(5-(2-(4-morpholinophenylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of ErbB2 by time resolved fluorescence assay | Bioorg Med Chem Lett 20: 2452-5 (2010) Article DOI: 10.1016/j.bmcl.2010.03.015 BindingDB Entry DOI: 10.7270/Q2M908VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022618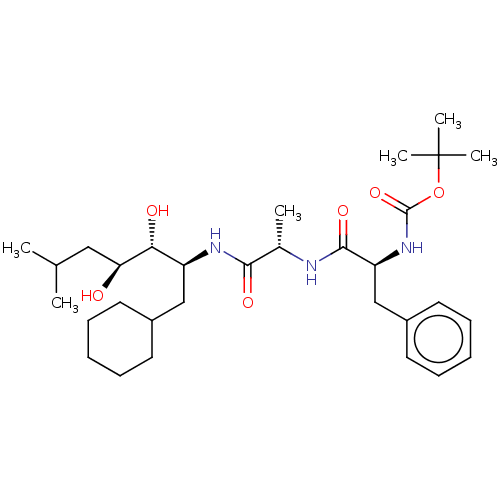 (CHEMBL3142283 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50174277 ((1R,2R,4S)-4-[(2R)-2-[(1R,9S,15R,16E,18R,19R,21R,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against FKBP12 | Bioorg Med Chem Lett 15: 5340-3 (2005) Article DOI: 10.1016/j.bmcl.2005.06.106 BindingDB Entry DOI: 10.7270/Q2V40TRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50315903 (CHEMBL1093234 | N-(3-(5-(2-(phenylamino)pyrimidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of ErbB2 by time resolved fluorescence assay | Bioorg Med Chem Lett 20: 2452-5 (2010) Article DOI: 10.1016/j.bmcl.2010.03.015 BindingDB Entry DOI: 10.7270/Q2M908VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50315897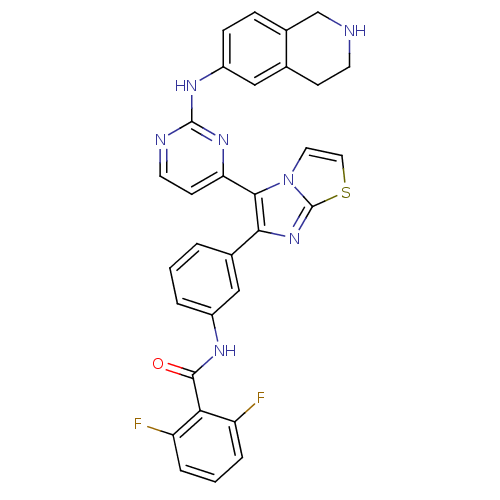 (2,6-difluoro-N-(3-(5-(2-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of ErbB2 by time resolved fluorescence assay | Bioorg Med Chem Lett 20: 2452-5 (2010) Article DOI: 10.1016/j.bmcl.2010.03.015 BindingDB Entry DOI: 10.7270/Q2M908VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022375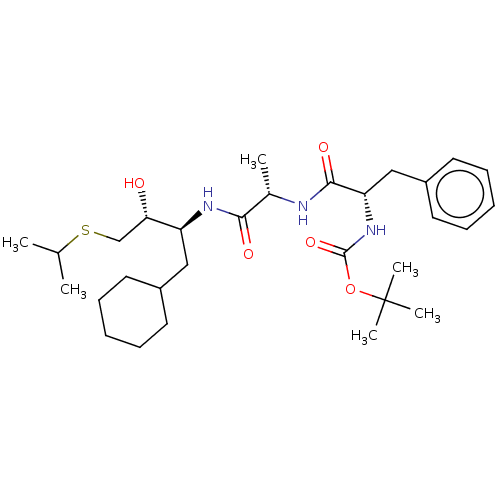 (BDBM50022873 | CHEMBL348469 | {1-[1-(1-Cyclohexylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human renal renin at pH 6 | J Med Chem 31: 532-9 (1988) BindingDB Entry DOI: 10.7270/Q2NK3D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17478 (2-benzenesulfonamido-5,6,7,8-tetrahydronaphthalene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | Bioorg Med Chem Lett 16: 3574-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.085 BindingDB Entry DOI: 10.7270/Q2TB1556 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17579 (2-({2-[3-(diethylamino)propyl]benzene}sulfonamido)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | J Med Chem 49: 3832-49 (2006) Article DOI: 10.1021/jm0601001 BindingDB Entry DOI: 10.7270/Q22V2DDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17573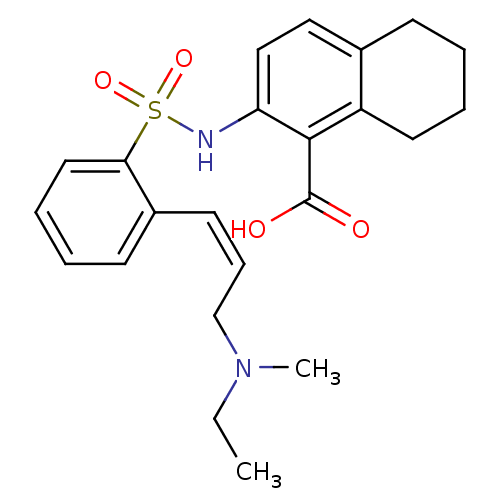 (2-({2-[(1Z)-3-[ethyl(methyl)amino]prop-1-en-1-yl]b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | J Med Chem 49: 3832-49 (2006) Article DOI: 10.1021/jm0601001 BindingDB Entry DOI: 10.7270/Q22V2DDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022592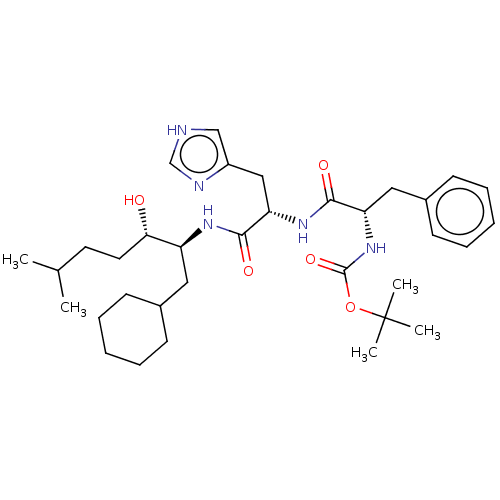 (CHEMBL3348547 | {1-[1-(1-Cyclohexylmethyl-2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022639 (CHEMBL3348539 | {1-[1-(1-Cyclohexylmethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity was tested against purified human renal renin | J Med Chem 31: 2264-76 (1989) BindingDB Entry DOI: 10.7270/Q2T72GDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17564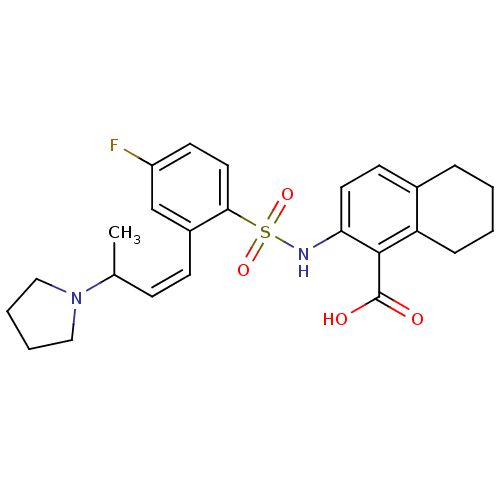 (2-({4-fluoro-2-[(1Z)-3-(pyrrolidin-1-yl)but-1-en-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | J Med Chem 49: 3832-49 (2006) Article DOI: 10.1021/jm0601001 BindingDB Entry DOI: 10.7270/Q22V2DDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17505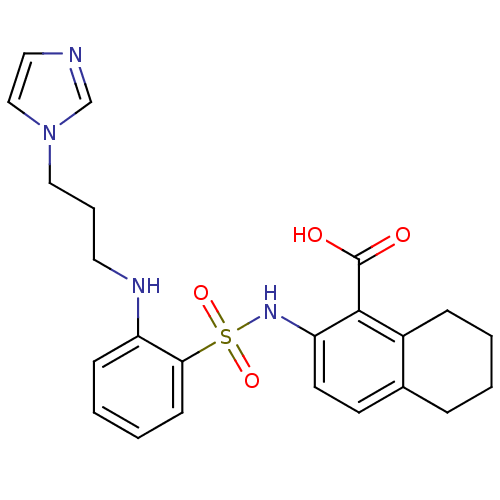 (2-[(2-{[3-(1H-imidazol-1-yl)propyl]amino}benzene)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | J Med Chem 49: 3832-49 (2006) Article DOI: 10.1021/jm0601001 BindingDB Entry DOI: 10.7270/Q22V2DDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17607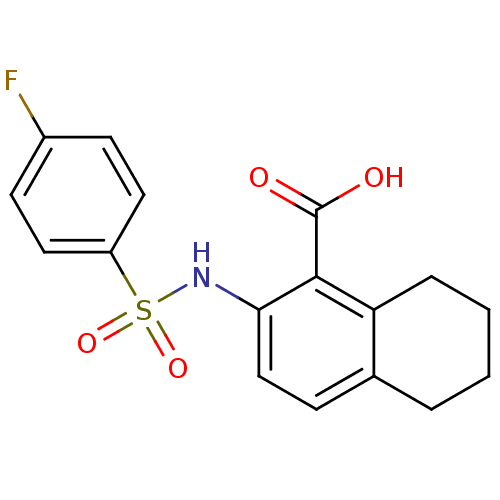 (2-[(4-fluorobenzene)sulfonamido]-5,6,7,8-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase-2 activity by monitoring the production of free methionine with... | Bioorg Med Chem Lett 16: 3574-7 (2006) Article DOI: 10.1016/j.bmcl.2006.03.085 BindingDB Entry DOI: 10.7270/Q2TB1556 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 588 total ) | Next | Last >> |