

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083672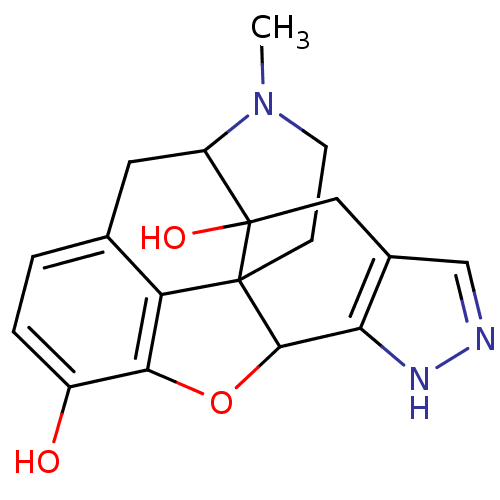 (18-methyl-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083671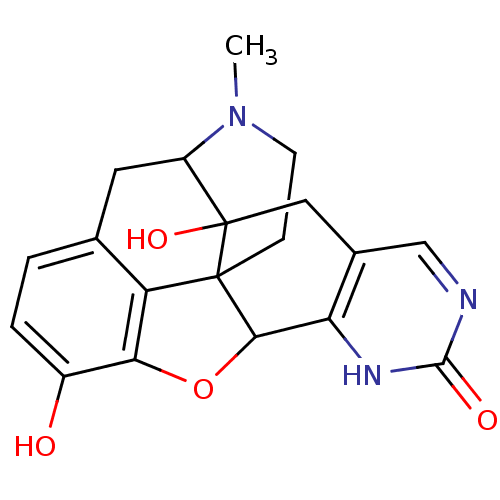 (19-methyl-11-oxa-6,8,19-triazahexacyclo[10.9.1.01,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083668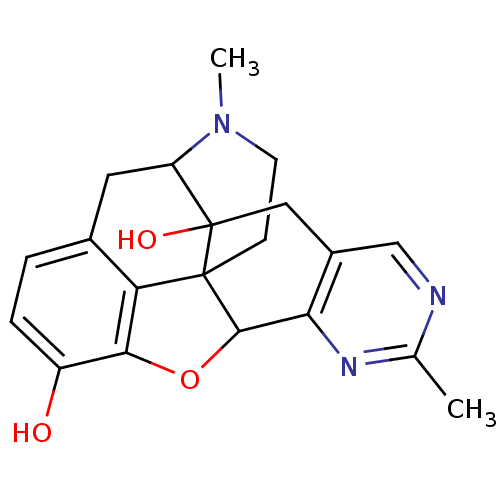 (7,19-dimethyl-11-oxa-6,8,19-triazahexacyclo[10.9.1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083670 (19-methyl-7-phenyl-11-oxa-6,8,19-triazahexacyclo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Delta receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-Cl-DPDPE as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083670 (19-methyl-7-phenyl-11-oxa-6,8,19-triazahexacyclo[1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083669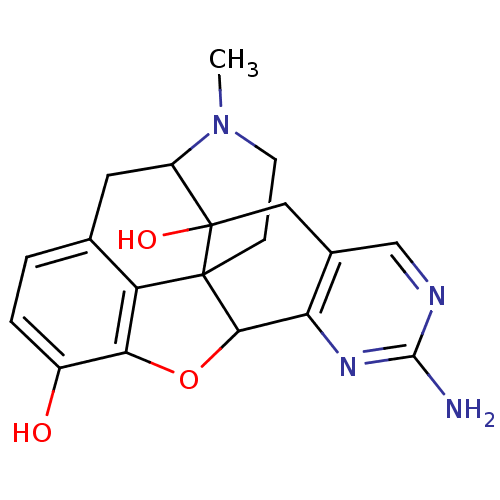 (7-amino-19-methyl-11-oxa-6,8,19-triazahexacyclo[10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083671 (19-methyl-11-oxa-6,8,19-triazahexacyclo[10.9.1.01,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonistic activity against human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083672 (18-methyl-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonistic activity against human opioid Mu receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-DAMGO as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083672 (18-methyl-10-oxa-6,7,18-triazahexacyclo[9.9.1.01,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Kappa receptor transfected into Chinese hamster ovary (CHO) cells using [3H]U69,593 as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083671 (19-methyl-11-oxa-6,8,19-triazahexacyclo[10.9.1.01,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Delta receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-Cl-DPDPE as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083669 (7-amino-19-methyl-11-oxa-6,8,19-triazahexacyclo[10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Delta receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-Cl-DPDPE as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083669 (7-amino-19-methyl-11-oxa-6,8,19-triazahexacyclo[10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Kappa receptor transfected into Chinese hamster ovary (CHO) cells using [3H]U69,593 as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083668 (7,19-dimethyl-11-oxa-6,8,19-triazahexacyclo[10.9.1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Delta receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-Cl-DPDPE as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057479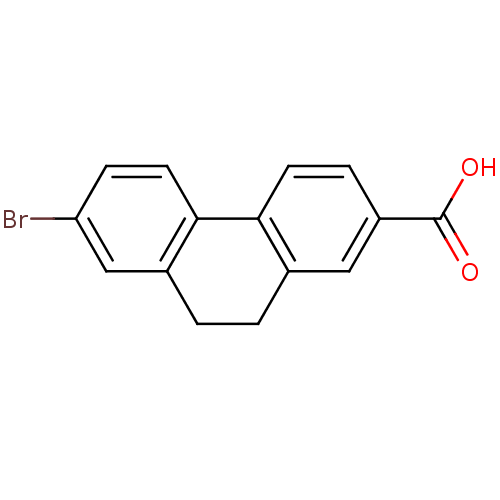 (7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroid 5-alpha-reductase type I | Bioorg Med Chem Lett 10: 1909-11 (2001) BindingDB Entry DOI: 10.7270/Q2M044PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083670 (19-methyl-7-phenyl-11-oxa-6,8,19-triazahexacyclo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards human opioid Kappa receptor transfected into Chinese hamster ovary (CHO) cells using [3H]U69,593 as radioligand. | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083668 (7,19-dimethyl-11-oxa-6,8,19-triazahexacyclo[10.9.1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonistic activity against human opioid Delta receptor transfected into Chinese hamster ovary (CHO) cells using [3H]-Cl-DPDPE as radioligand | Bioorg Med Chem Lett 9: 3375-80 (2000) BindingDB Entry DOI: 10.7270/Q2XP7440 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50213272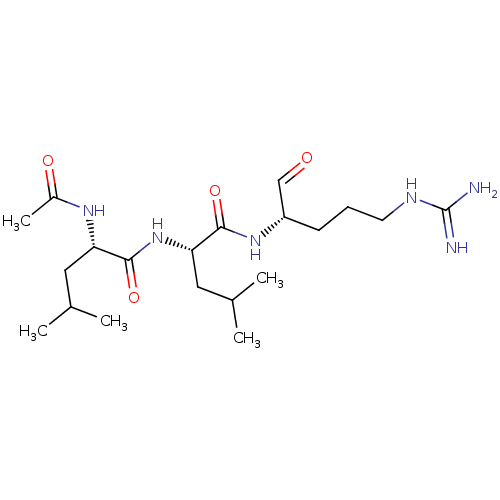 (CHEBI:6426 | Leupeptin) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain using cbzFRamc substrate preincubated for 10 mins followed by addition of substrate and measured every 30 sec for 60 mins... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00201 BindingDB Entry DOI: 10.7270/Q2JW8JJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50553988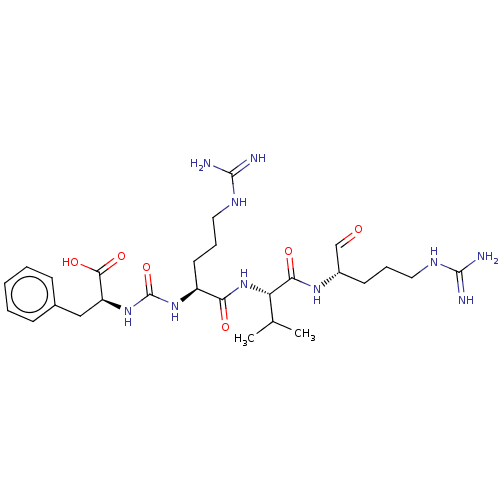 (Antipain) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain using cbzFRamc substrate preincubated for 10 mins followed by addition of substrate and measured every 30 sec for 60 mins... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00201 BindingDB Entry DOI: 10.7270/Q2JW8JJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070046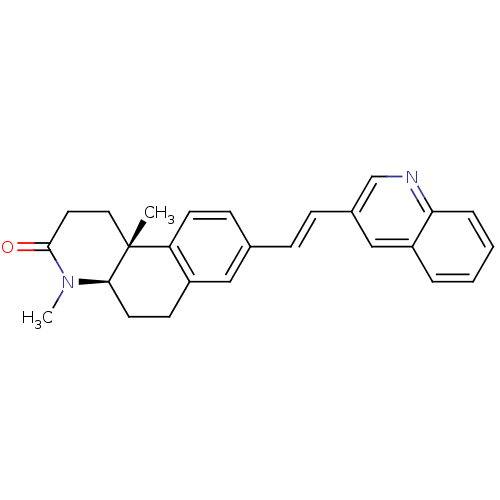 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-2-quinolin-3-yl-v...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 1 enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070049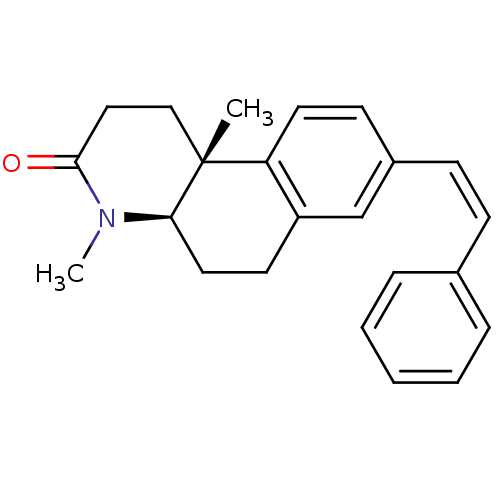 ((4aR,10bR)-4,10b-Dimethyl-8-((Z)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50553989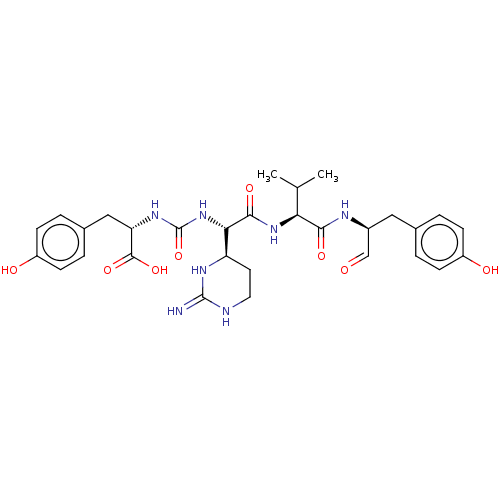 (CHEMBL4764505) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain using cbzFRamc substrate preincubated for 10 mins followed by addition of substrate and measured every 30 sec for 60 mins... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00201 BindingDB Entry DOI: 10.7270/Q2JW8JJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368782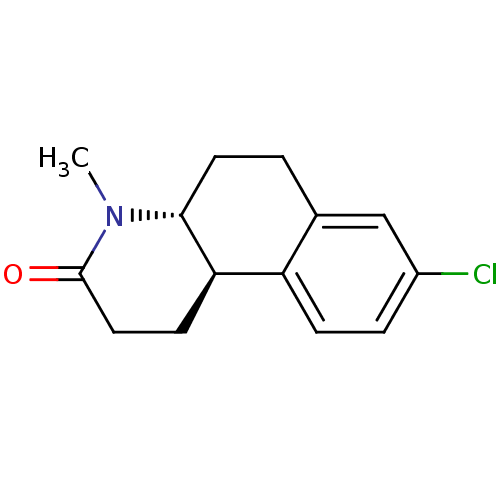 (Bexlosteride | CHEMBL24955 | LY-191704) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50031890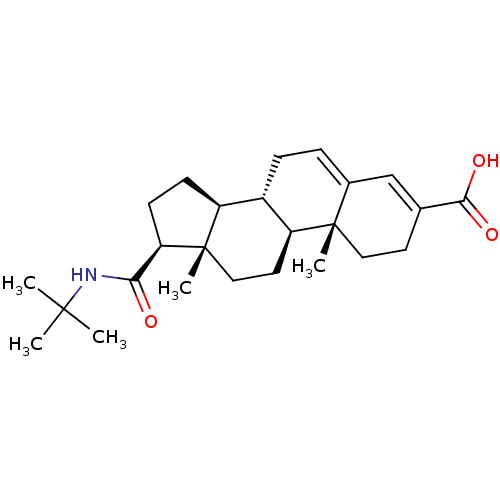 ((10R,13S)-17-tert-Butylcarbamoyl-10,13-dimethyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of Type II 5-alpha-reductase in Human Prostate Homogenates (HPH) | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559431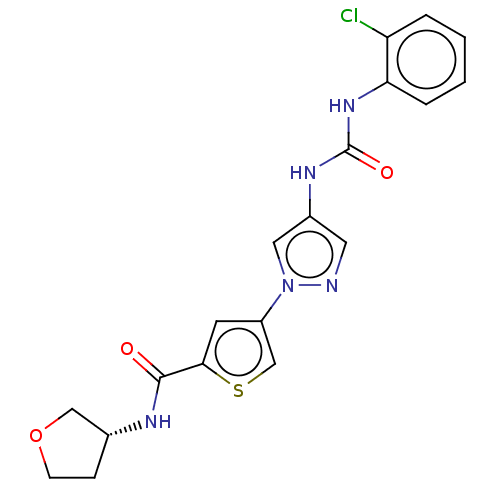 (CHEMBL4753310) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of Type II 5-alpha-reductase in Human Prostate Homogenates (HPH). | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044878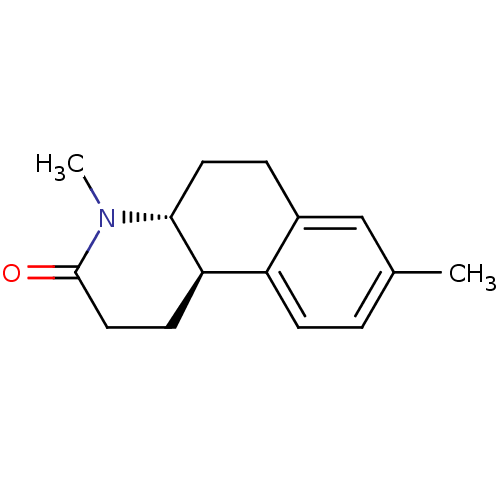 (4,8-Dimethyl-1,4,4a,5,6,10b-hexahydro-2H-benzo[f]q...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559434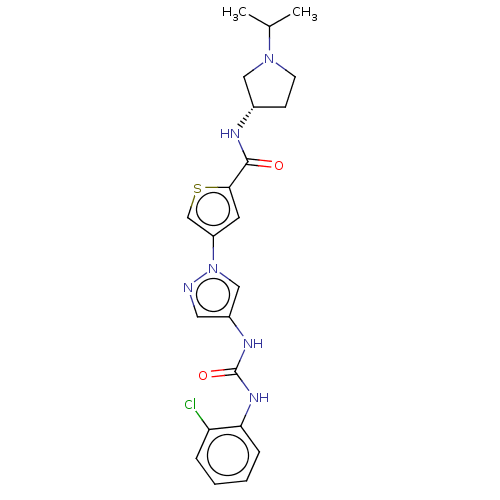 (CHEMBL4784095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044879 ((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroid 5-alpha-reductase type I | Bioorg Med Chem Lett 10: 1909-11 (2001) BindingDB Entry DOI: 10.7270/Q2M044PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044879 ((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50553990 (CHEMBL4788595) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of papaya papain using cbzFRamc substrate preincubated for 10 mins followed by addition of substrate and measured every 30 sec for 60 mins... | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00201 BindingDB Entry DOI: 10.7270/Q2JW8JJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070054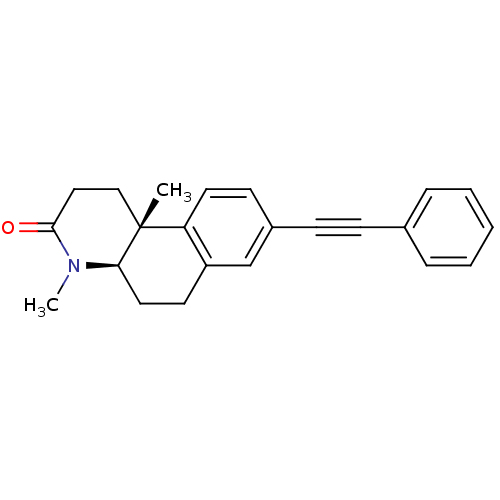 ((4aR,10bR)-4,10b-Dimethyl-8-phenylethynyl-1,4,4a,5...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070051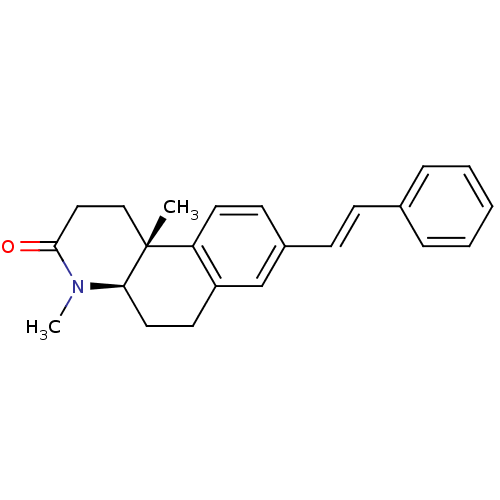 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury Curated by ChEMBL | Assay Description In vitro inhibitory activity against Steroid 5-alpha-reductase type I | Bioorg Med Chem Lett 10: 1909-11 (2001) BindingDB Entry DOI: 10.7270/Q2M044PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070051 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559423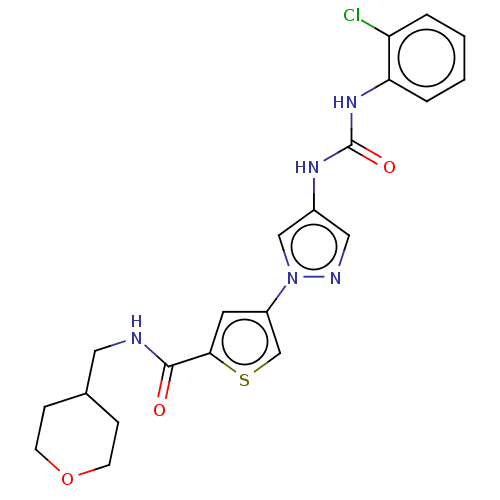 (CHEMBL4744992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044881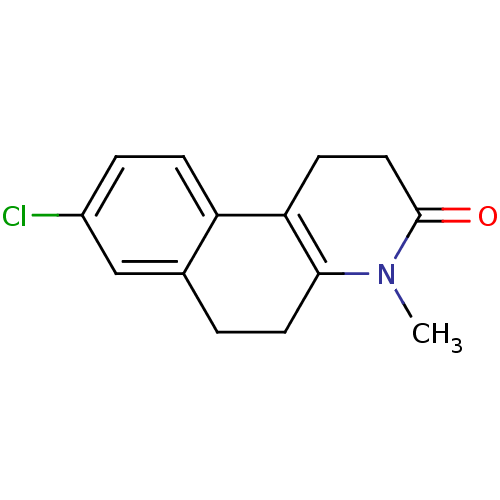 (8-Chloro-4-methyl-1,4,5,6-tetrahydro-2H-benzo[f]qu...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070044 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-2-quinolin-2-yl-v...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 1 enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559422 (CHEMBL4778645) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559430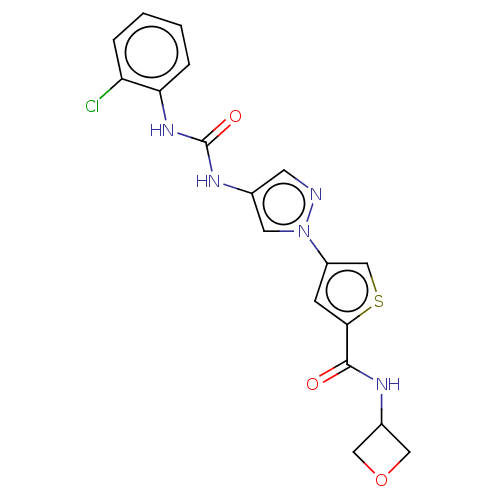 (CHEMBL4753641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044891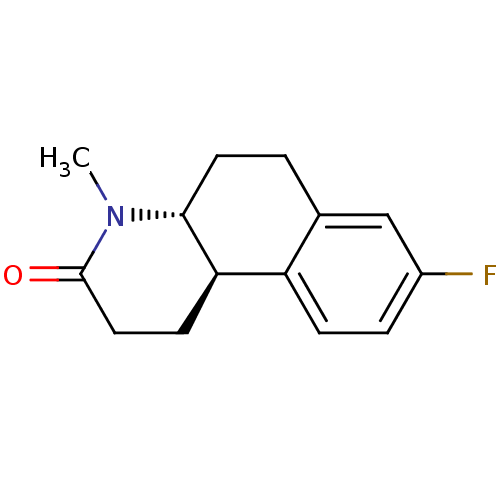 (8-Fluoro-4-methyl-1,4,4a,5,6,10b-hexahydro-2H-benz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559435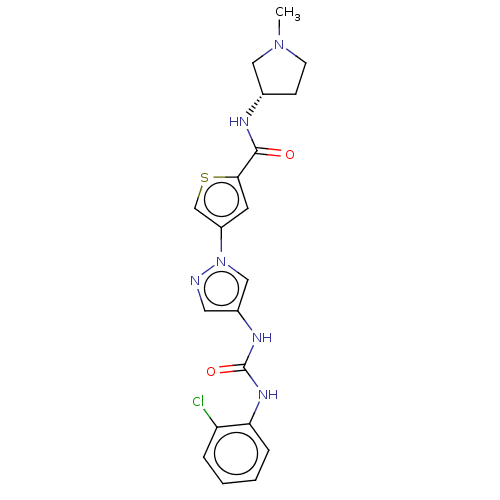 (CHEMBL4754584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044885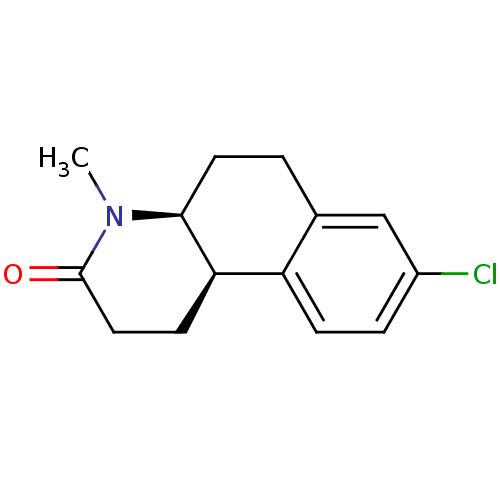 ((4aS,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559429 (CHEMBL4758113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559441 (CHEMBL4783382) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559432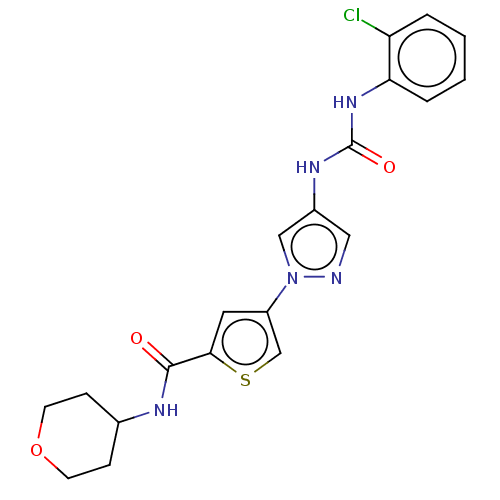 (CHEMBL4762965) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50070054 ((4aR,10bR)-4,10b-Dimethyl-8-phenylethynyl-1,4,4a,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 2 as [3H]-T to [3H]-DHT conversion human prostate nuclear membrane | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559419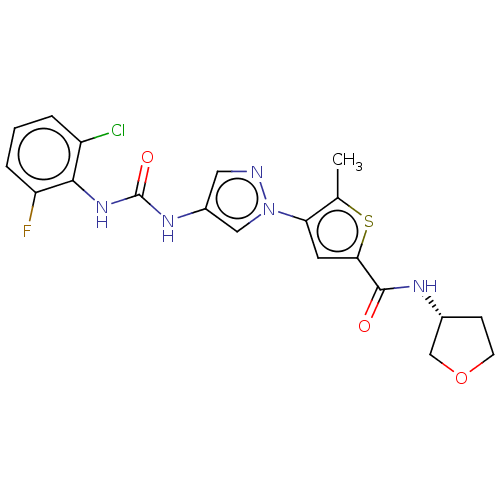 (CHEMBL4749547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50559421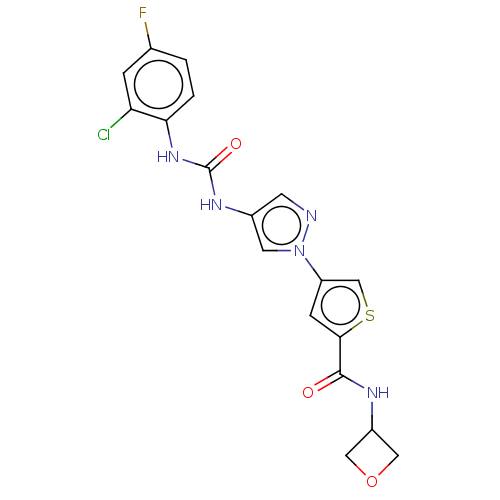 (CHEMBL4754908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using ATF2 as substrate after 22 mins in presence of ATP by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00533 BindingDB Entry DOI: 10.7270/Q2KW5KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070047 ((4aR,10bR)-8-Furan-2-yl-4,10b-dimethyl-1,4,4a,5,6,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 1 enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044886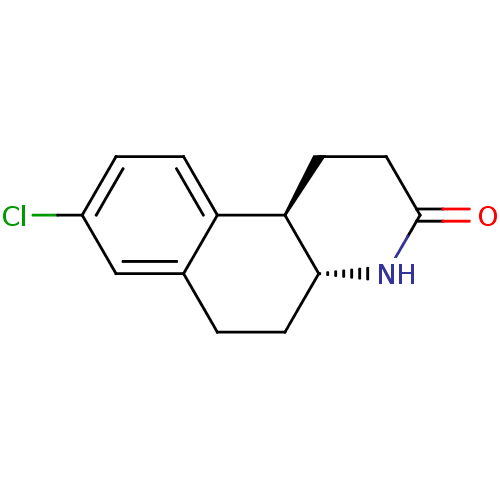 (8-Chloro-1,4,4a,5,6,10b-hexahydro-2H-benzo[f]quino...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of Type I 5-alpha-reductase in Human genital skin (Hs68) foreskin fibroblast cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 213 total ) | Next | Last >> |