

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239934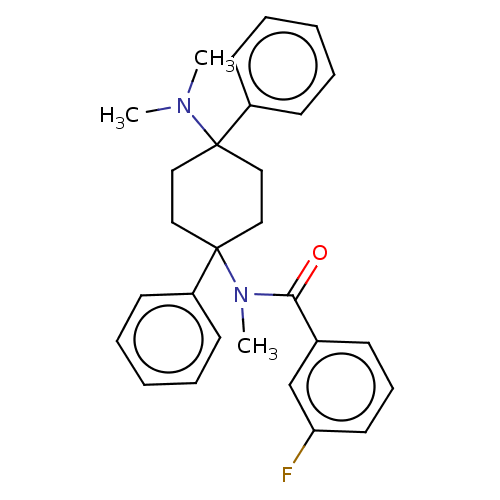 (US9403767, 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021214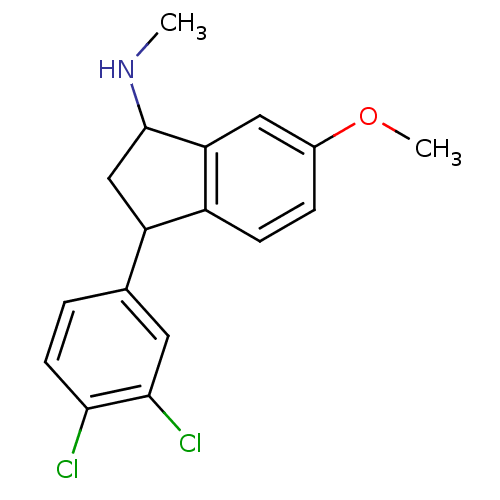 (CHEMBL300019 | CHEMBL537996 | [3-(3,4-Dichloro-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239934 (US9403767, 117) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239911 (US9403767, 76 | US9403767, 77) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021226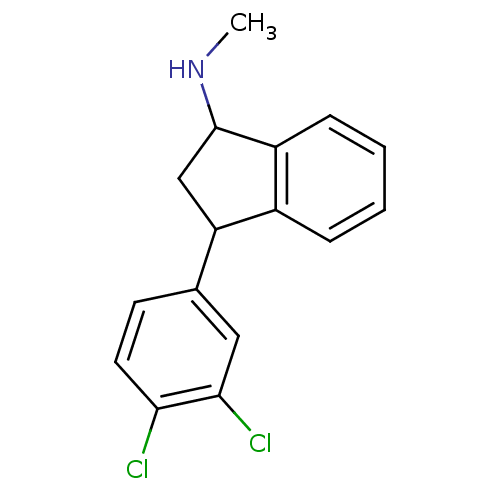 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50330860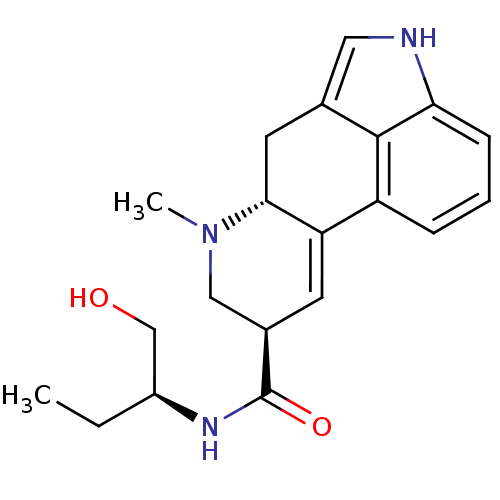 (CHEMBL1201356 | METHYLERGONOVINE | Methylergometri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Circulation 102: 2836-41 (2000) Article DOI: 10.1161/01.cir.102.23.2836 BindingDB Entry DOI: 10.7270/Q2H70DD1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239921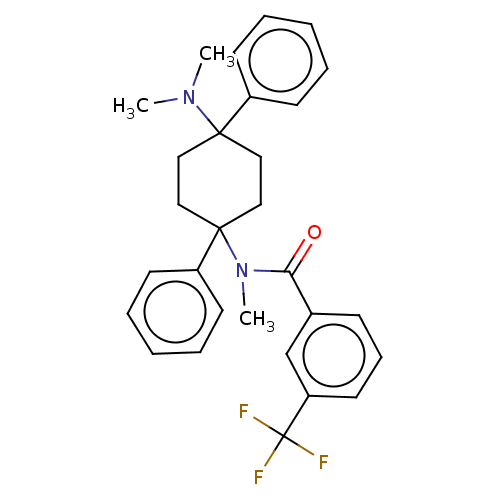 (US9403767, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239925 (US9403767, 104 | US9403767, 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50146043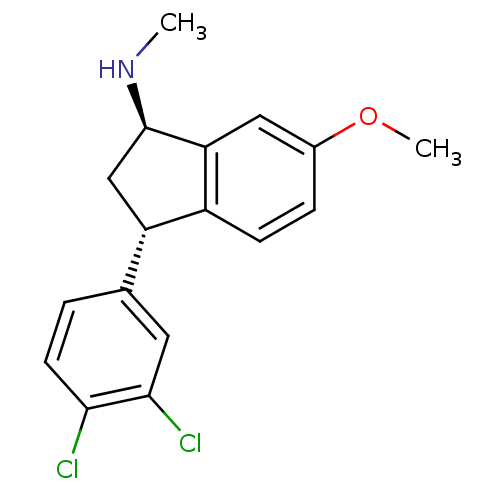 (CHEMBL543372 | [(1R,3S)-3-(3,4-Dichloro-phenyl)-6-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for DA transporter using [125I]RTI-55 in frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50146042 (1-(3,4-Dichloro-phenyl)-3-diethylamino-indan-5-ol ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239911 (US9403767, 76 | US9403767, 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22170 ((2S)-1-(4-{2-[bis(4-fluorophenyl)methoxy]ethyl}pip...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239914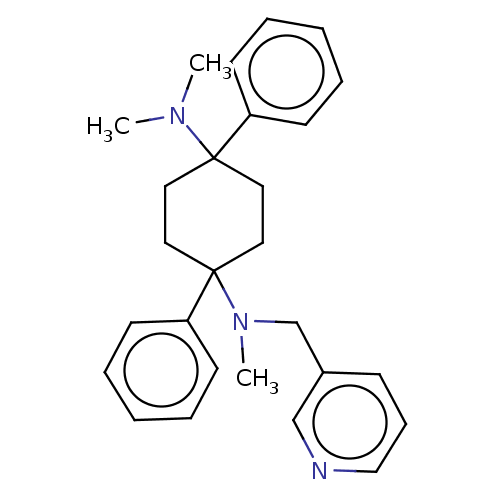 (US9403767, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239867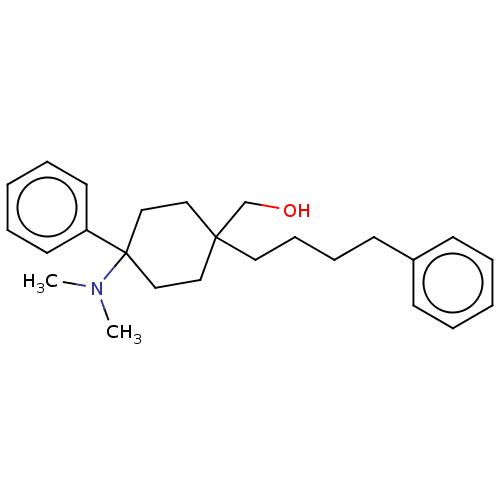 (US9403767, 20 | US9403767, 21) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021214 (CHEMBL300019 | CHEMBL537996 | [3-(3,4-Dichloro-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of from norepinephrine transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239921 (US9403767, 99) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239925 (US9403767, 104 | US9403767, 105) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239887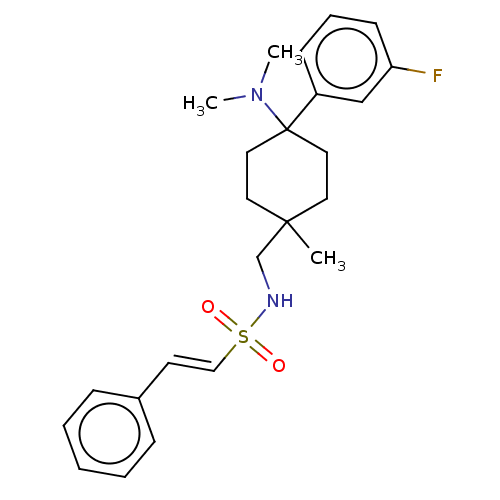 (US9403767, 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.24 | -50.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239865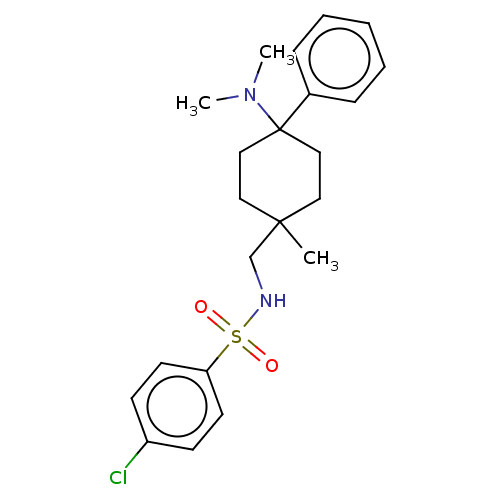 (US9403767, 18) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.25 | -50.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239892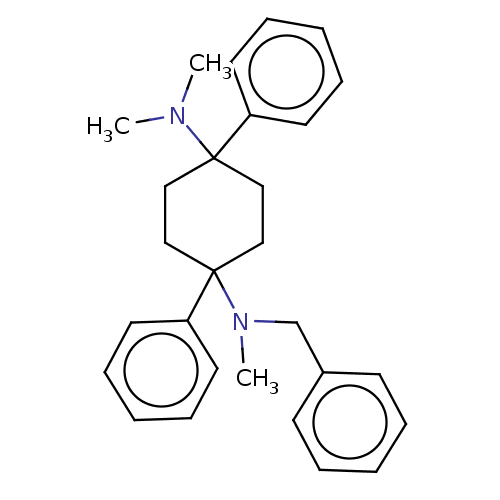 (US9403767, 50 | US9403767, 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239935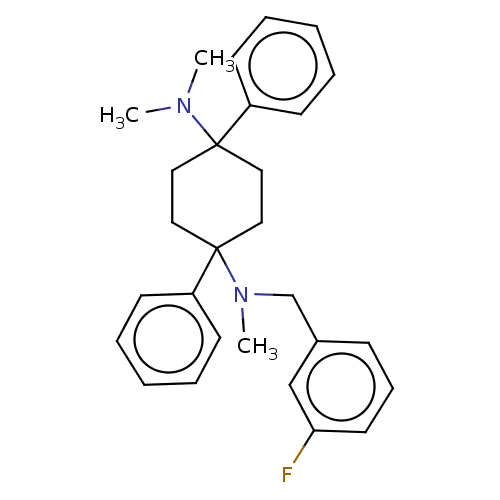 (US9403767, 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239873 (US9403767, 29 | US9403767, 30) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239890 (US9403767, 48 | US9403767, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239873 (US9403767, 29 | US9403767, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50021226 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for DA transporter using [125I]RTI-55 in frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50031942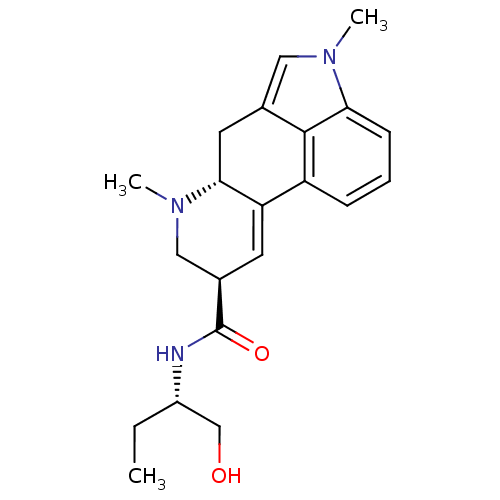 ((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Circulation 102: 2836-41 (2000) Article DOI: 10.1161/01.cir.102.23.2836 BindingDB Entry DOI: 10.7270/Q2H70DD1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239884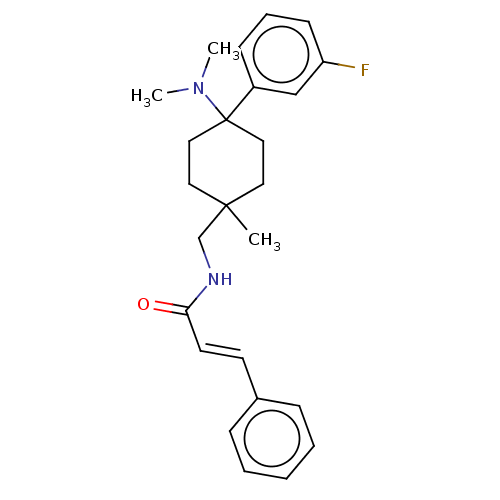 (US9403767, 42) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | -49.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50021226 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50146040 ((1R,3S)-1-(3,4-Dichloro-phenyl)-3-methylamino-inda...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50146040 ((1R,3S)-1-(3,4-Dichloro-phenyl)-3-methylamino-inda...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Reuptake inhibitory activity against Serotonin transporter | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50021226 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Reuptake inhibitory activity against dopamine DA transporter | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50083215 ((+/-)-3-(4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50005572 ((3S,8R)-3-(4-Amino-phenyl)-8-methyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239873 (US9403767, 29 | US9403767, 30) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50110814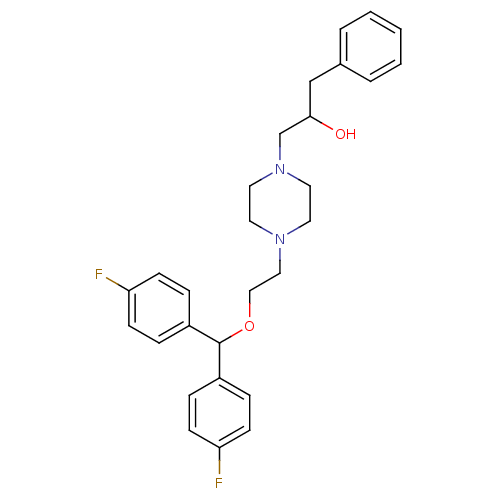 ((+/-)-1-(4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22172 ((2S)-1-{4-[2-(diphenylmethoxy)ethyl]piperazin-1-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM25870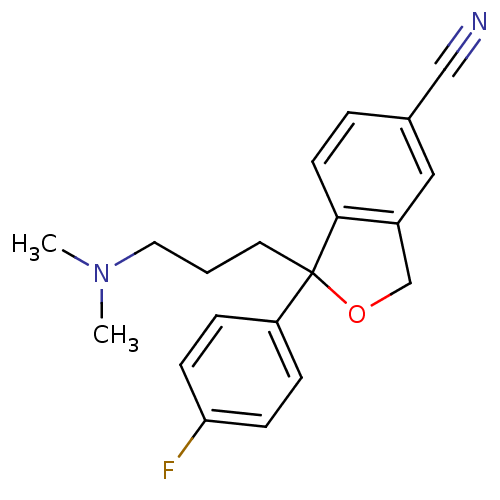 (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239923 (US9403767, 101 | US9403767, 102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239865 (US9403767, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Norepinephrine transporter (RAT) | BDBM50005536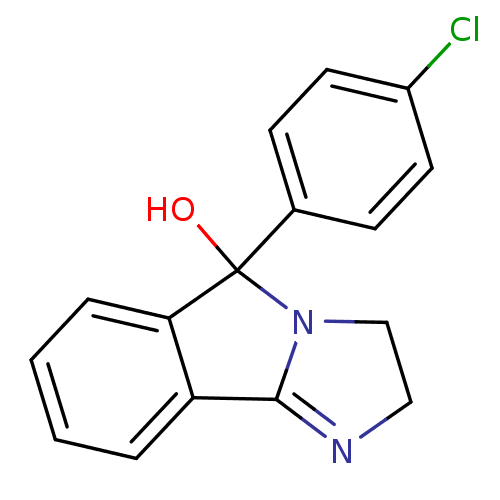 (42-548 | 5-(4-Chloro-phenyl)-2,5-dihydro-3H-imidaz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021213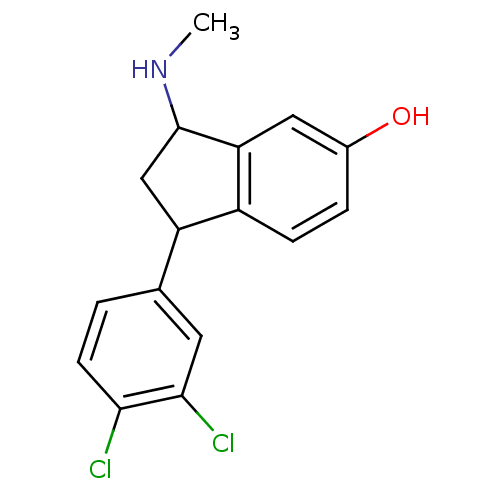 (1-(3,4-Dichloro-phenyl)-3-methylamino-indan-5-ol |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021226 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Reuptake inhibitory activity against Serotonin transporter | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50146040 ((1R,3S)-1-(3,4-Dichloro-phenyl)-3-methylamino-inda...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Reuptake inhibitory activity against dopamine DA transporter | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50027065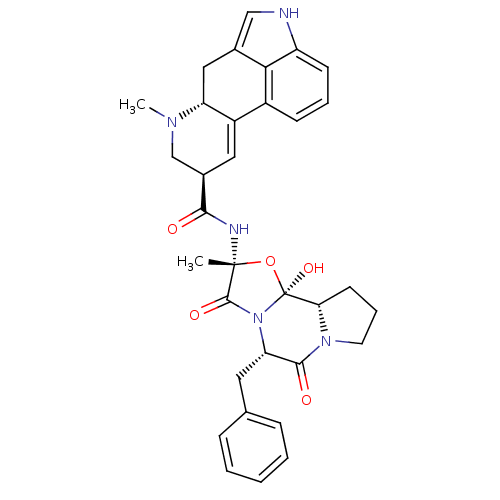 ((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Circulation 102: 2836-41 (2000) Article DOI: 10.1161/01.cir.102.23.2836 BindingDB Entry DOI: 10.7270/Q2H70DD1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM239947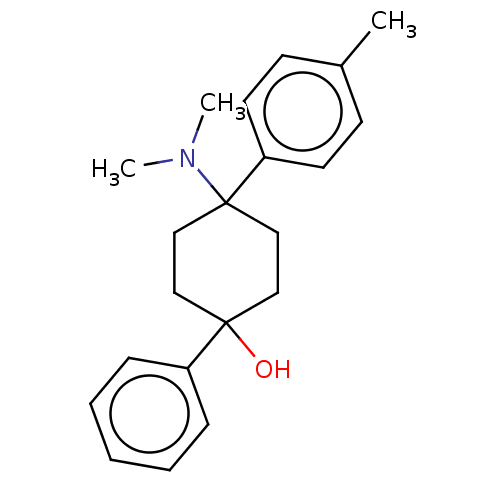 (US9403767, C-2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
GRUENENTHAL GMBH US Patent | Assay Description The affinity to the human μ-opiate receptor was determined in a homogeneous preparation in microtiter plates. For this, dilution series of the res... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22169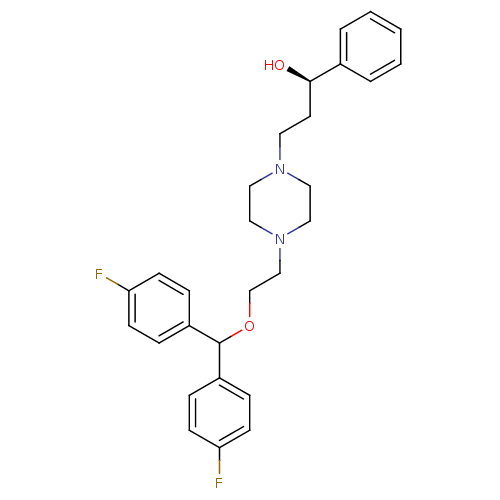 ((1R)-3-(4-{2-[bis(4-fluorophenyl)methoxy]ethyl}pip...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021226 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Synapse 39: 32-41 (2001) Article DOI: 10.1002/1098-2396(20010101)39:1 BindingDB Entry DOI: 10.7270/Q2NV9GT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50001915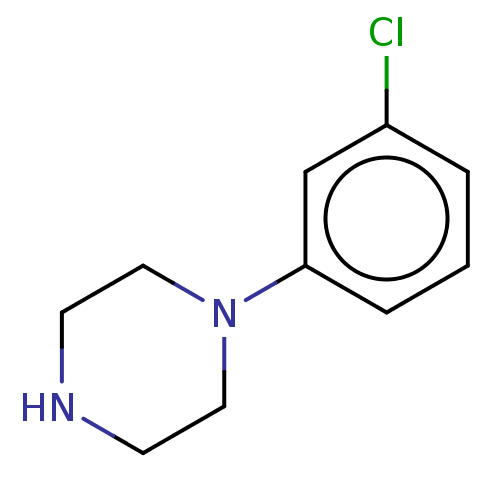 (1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by PDSP Ki Database | Circulation 102: 2836-41 (2000) Article DOI: 10.1161/01.cir.102.23.2836 BindingDB Entry DOI: 10.7270/Q2H70DD1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1209 total ) | Next | Last >> |