

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acidic mammalian chitinase (Mus musculus) | BDBM50243745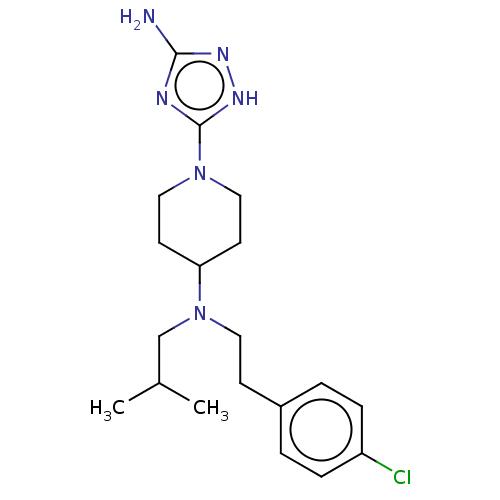 (CHEMBL4076989) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase, mitochondrial (Homo sapiens (Human)) | BDBM50121445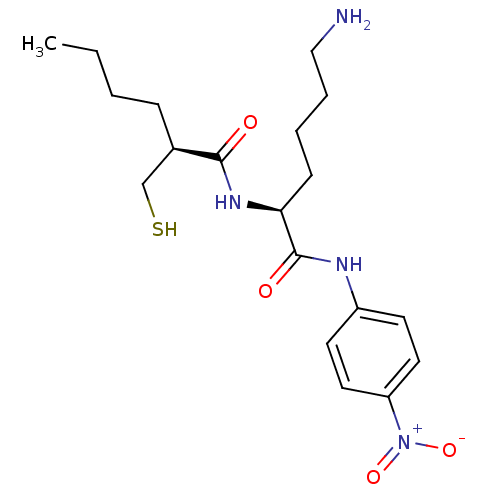 ((S)-2-Mercaptomethyl-hexanoic acid [(S)-5-amino-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptide deformylase, PDF.Fe of Escherichia coli | Bioorg Med Chem Lett 12: 3595-9 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase, mitochondrial (Homo sapiens (Human)) | BDBM50121442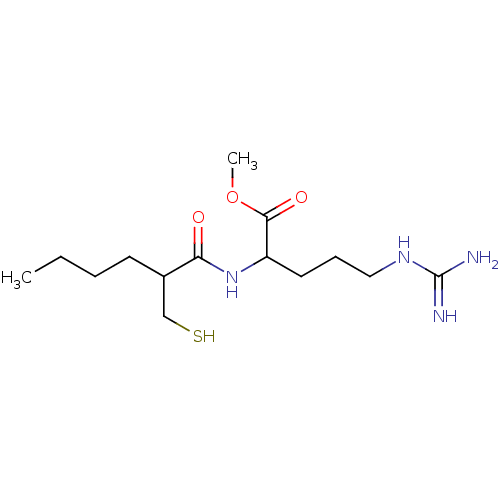 (5-Guanidino-2-(2-mercaptomethyl-hexanoylamino)-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli | Bioorg Med Chem Lett 12: 3595-9 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50243745 (CHEMBL4076989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290436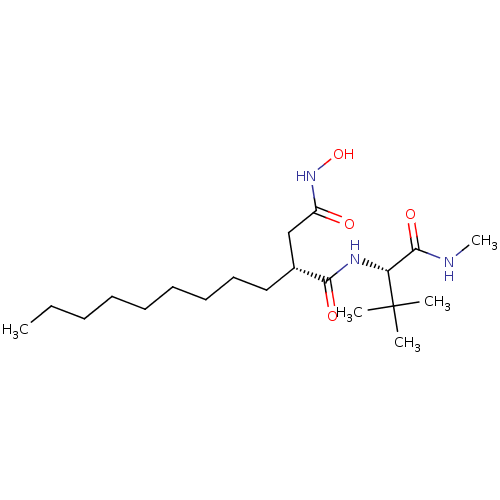 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50031790 ((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137332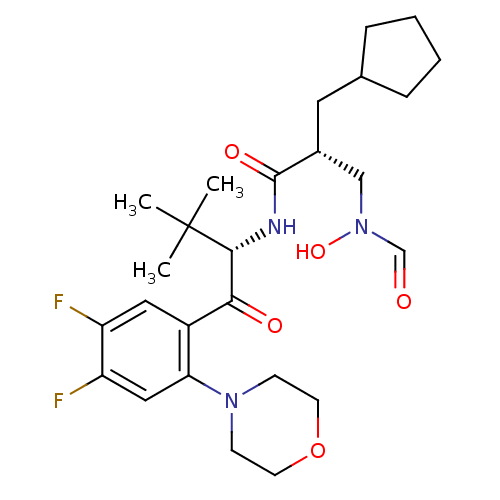 ((R)-2-Cyclopentylmethyl-N-[(S)-1-(4,5-difluoro-2-m...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290448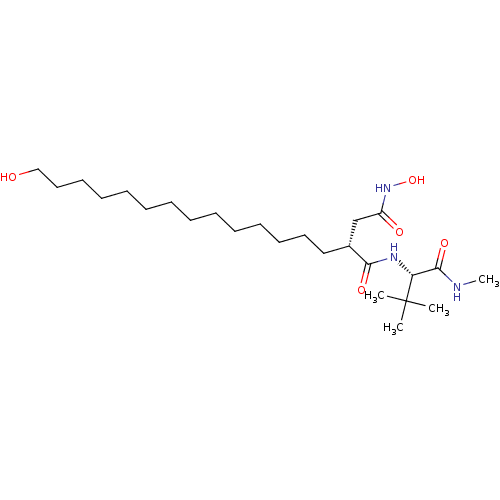 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290441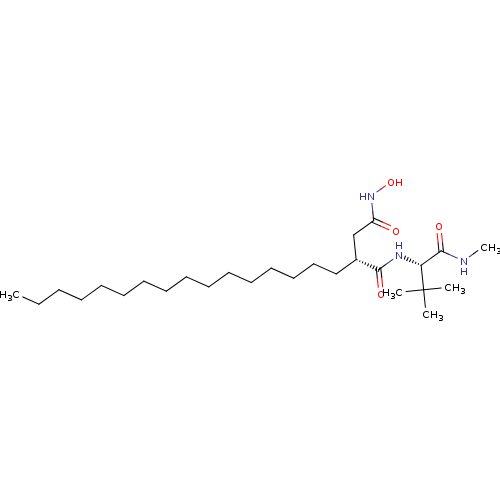 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290442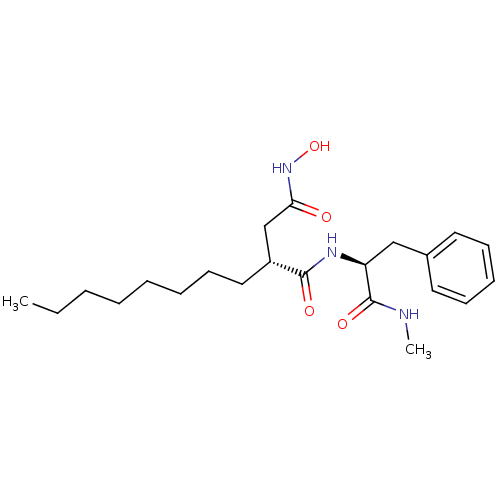 ((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137327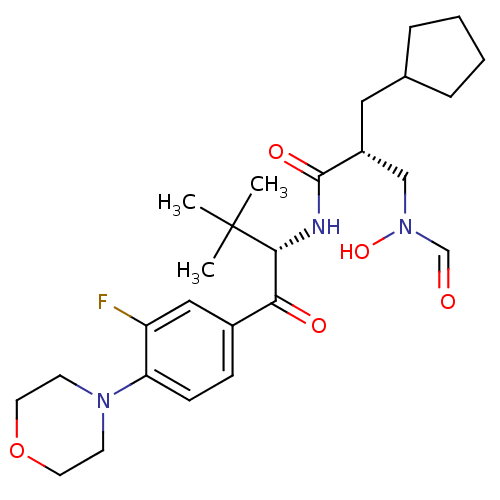 ((R)-2-Cyclopentylmethyl-N-[(S)-1-(3-fluoro-4-morph...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290439 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290449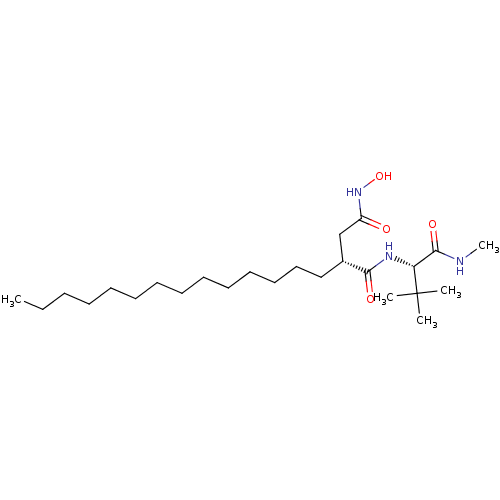 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290450 ((R)-2-Dodecyl-N*4*-hydroxy-N*1*-((S)-1-methylcarba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290456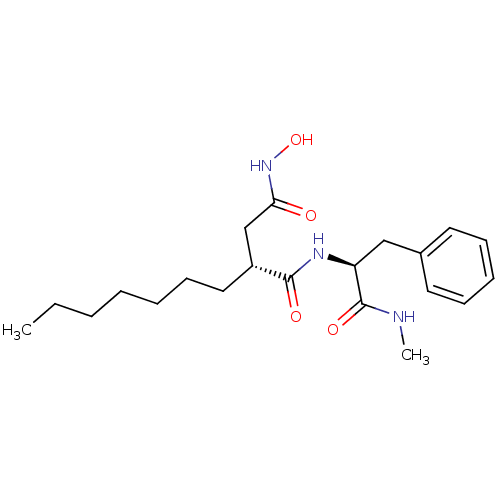 ((R)-2-Heptyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290433 ((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase, mitochondrial (Homo sapiens (Human)) | BDBM50121444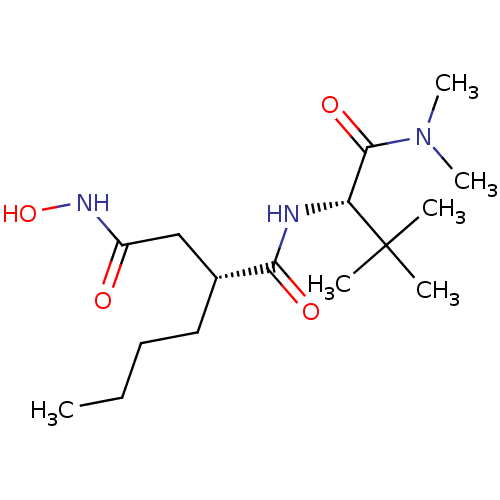 ((R)-2-Butyl-N*1*-((S)-1-dimethylcarbamoyl-2,2-dime...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli | Bioorg Med Chem Lett 12: 3595-9 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137345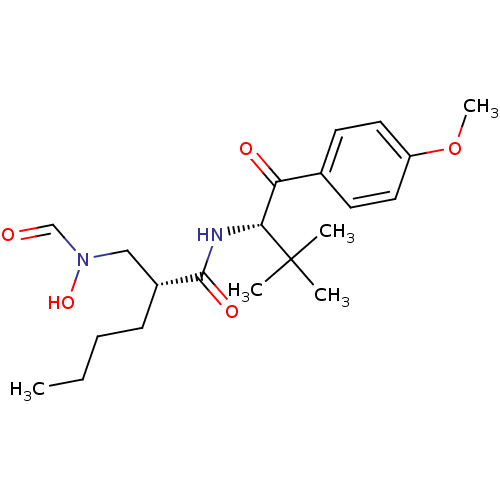 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137341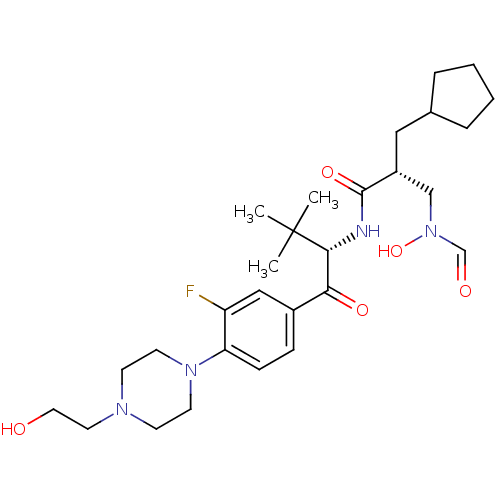 ((R)-2-Cyclopentylmethyl-N-((S)-1-{3-fluoro-4-[4-(2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137324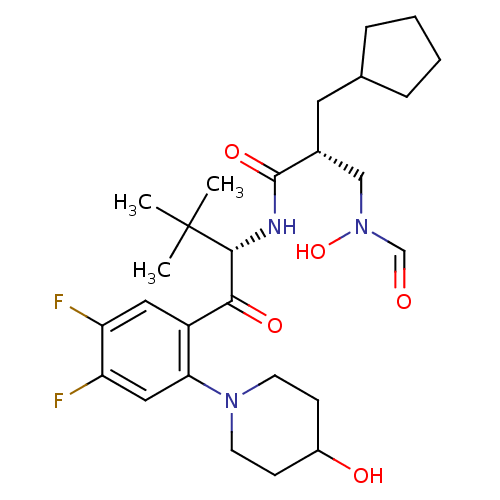 ((R)-2-Cyclopentylmethyl-N-{(S)-1-[4,5-difluoro-2-(...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50290436 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant Gelatinase B (MMP-9) | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of Coll-3 MMP-13 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50081851 ((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of HFC MMP-1 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137334 ((R)-2-Cyclopentylmethyl-N-((S)-2,2-dimethyl-1-{4-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137328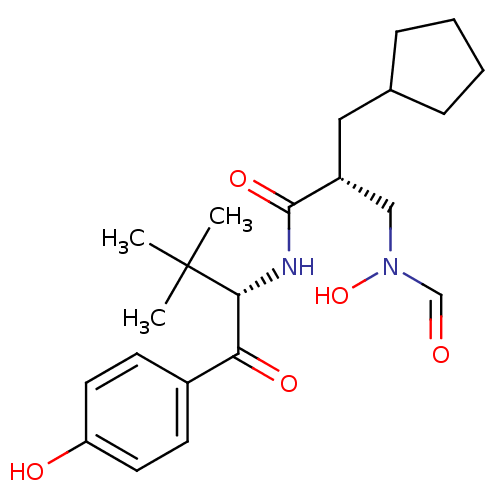 ((R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-amino)-N...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137320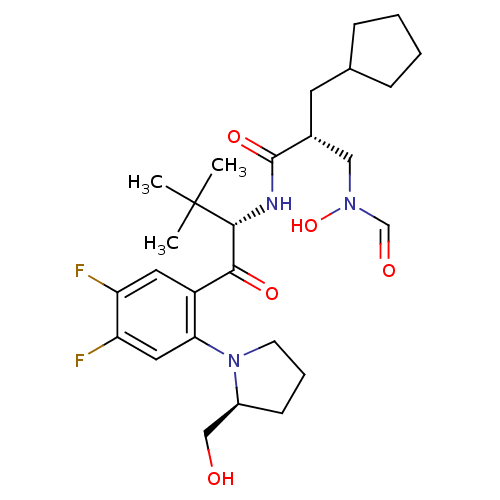 ((R)-2-Cyclopentylmethyl-N-{(S)-1-[4,5-difluoro-2-(...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of HNC MMP-8 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50081851 ((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of HNC MMP-8 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137326 ((R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-amino)-N...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137342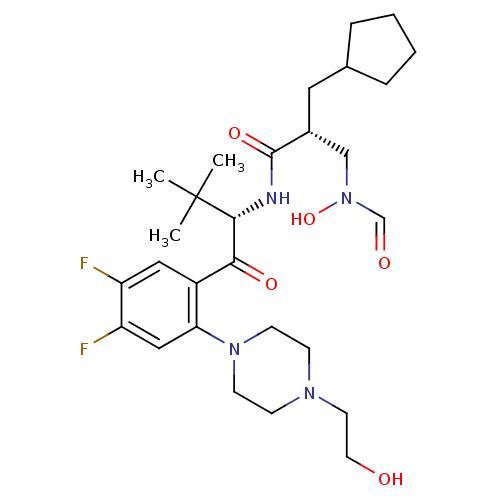 ((R)-2-Cyclopentylmethyl-N-((S)-1-{4,5-difluoro-2-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137339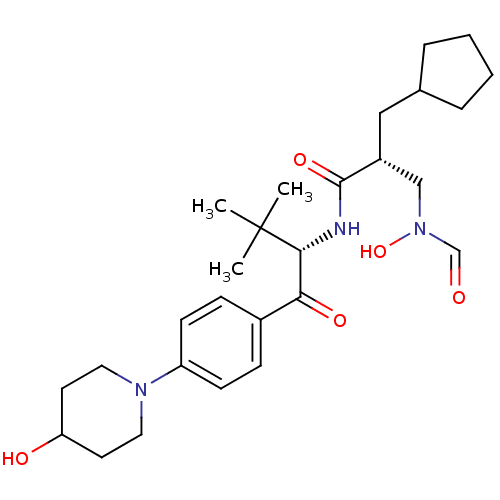 ((R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-amino)-N...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137329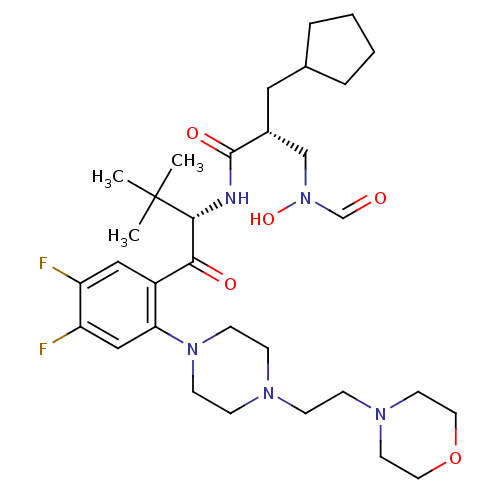 ((R)-2-Cyclopentylmethyl-N-((S)-1-{4,5-difluoro-2-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137316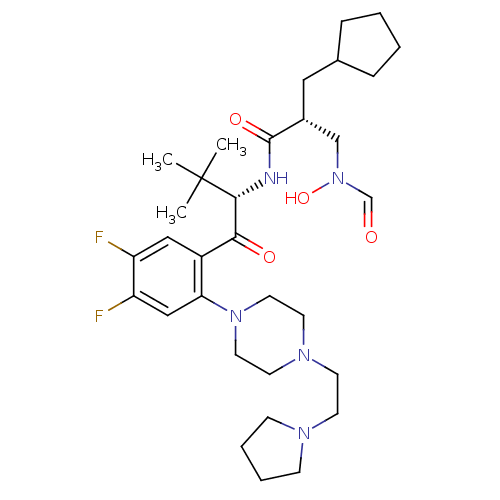 ((R)-2-Cyclopentylmethyl-N-((S)-1-{4,5-difluoro-2-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137333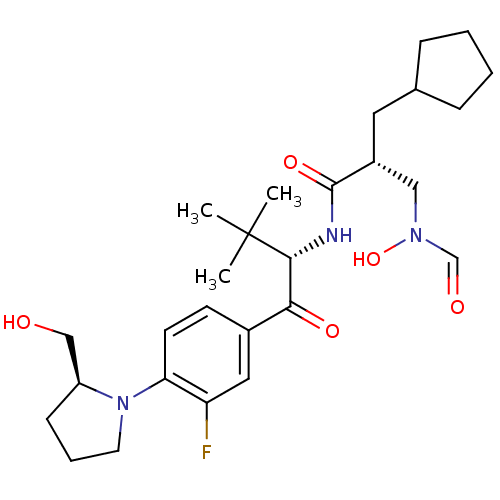 ((R)-2-Cyclopentylmethyl-N-{(S)-1-[3-fluoro-4-((S)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137314 ((R)-2-Cyclopentylmethyl-N-{(S)-1-[3-fluoro-4-(4-hy...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290453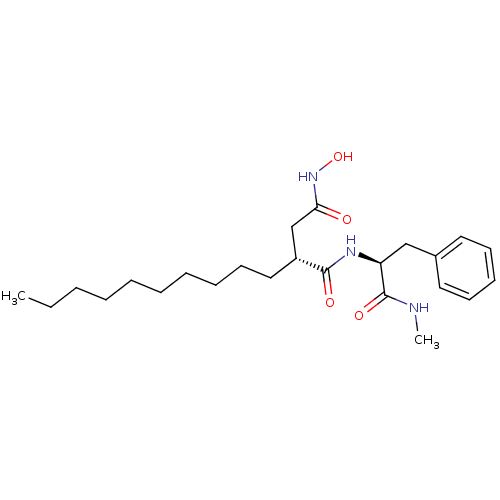 ((R)-2-Decyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137323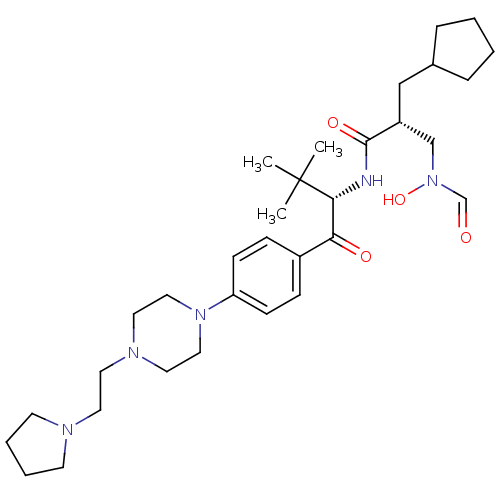 ((R)-2-Cyclopentylmethyl-N-((S)-2,2-dimethyl-1-{4-[...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137318 ((R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-amino)-N...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137346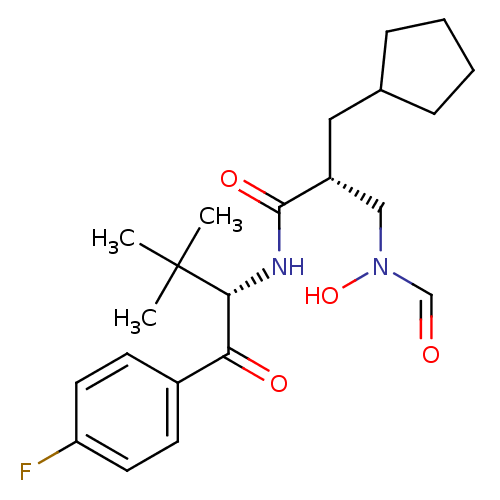 ((R)-2-Cyclopentylmethyl-N-[(S)-1-(4-fluoro-benzoyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137330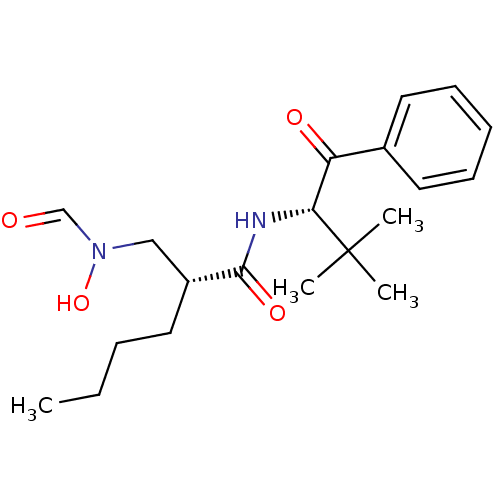 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50290440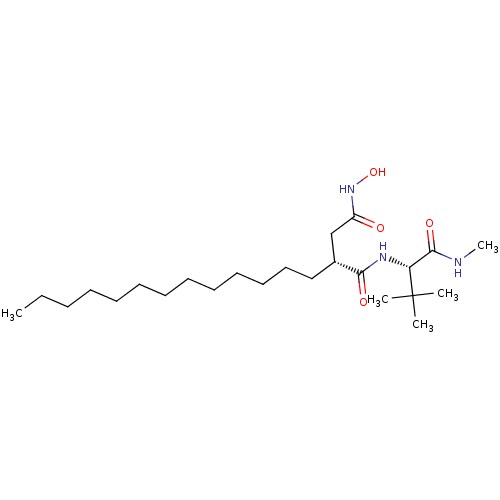 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50081869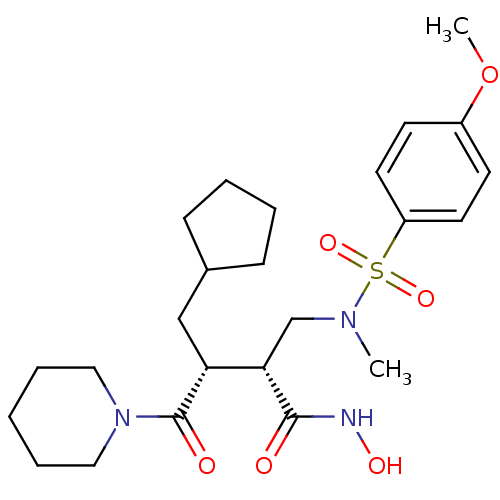 ((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of HNC MMP-8 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137348 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50137319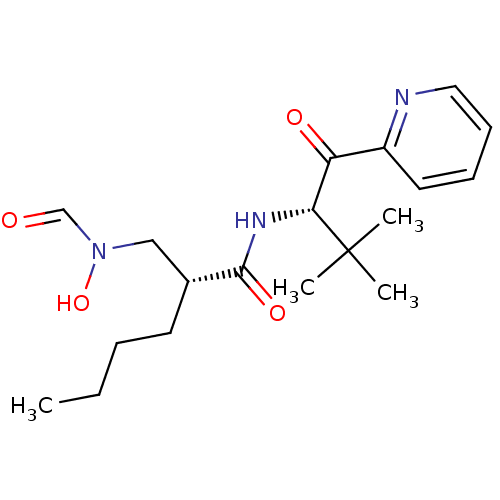 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli peptide deformylase (PDF) Nickel containing enzyme | Bioorg Med Chem Lett 14: 59-62 (2003) BindingDB Entry DOI: 10.7270/Q2T43SHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50081866 ((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of HNC MMP-8 | Bioorg Med Chem Lett 9: 2887-92 (1999) BindingDB Entry DOI: 10.7270/Q25H7FGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243685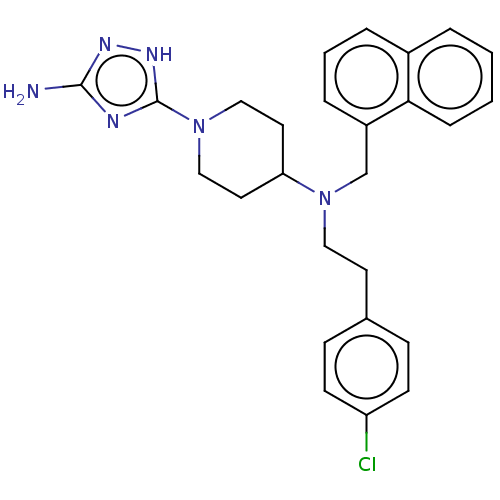 (CHEMBL4084573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243795 (CHEMBL4077644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). | Bioorg Med Chem Lett 7: 193-198 (1997) Article DOI: 10.1016/S0960-894X(96)00602-6 BindingDB Entry DOI: 10.7270/Q2057FXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 521 total ) | Next | Last >> |