

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50195783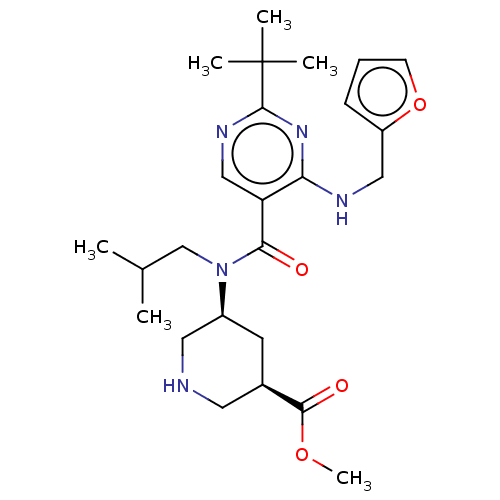 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195783 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273203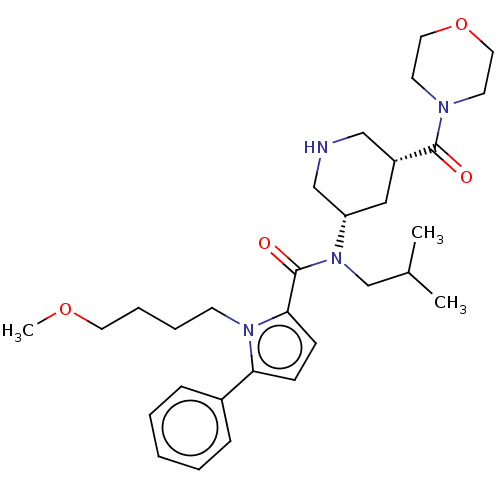 (CHEMBL4128929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273163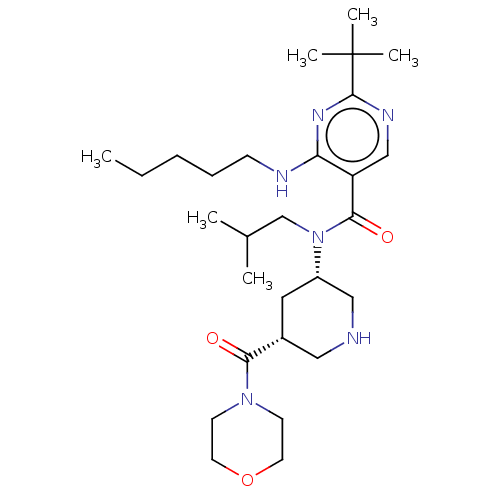 (CHEMBL4126136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273198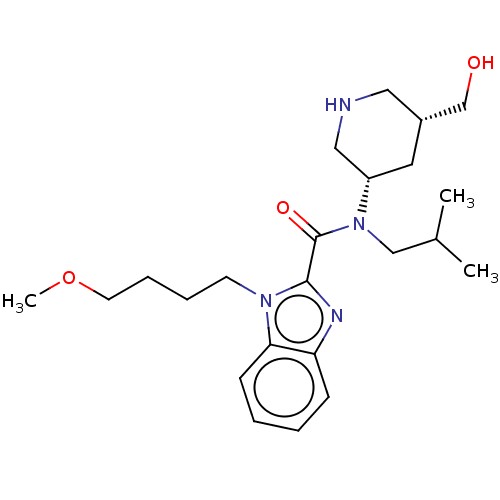 (CHEMBL4130200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273198 (CHEMBL4130200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195783 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195783 (CHEMBL3907708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195782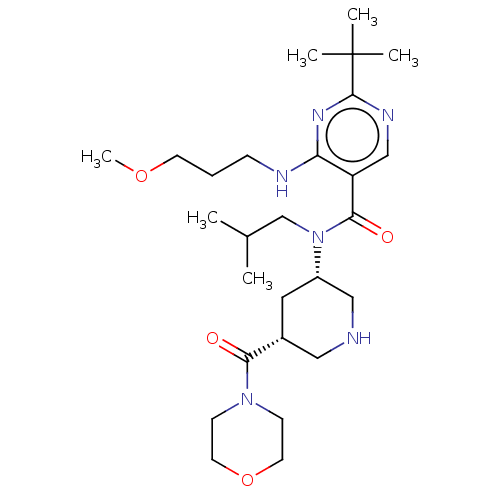 (CHEMBL3958809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273202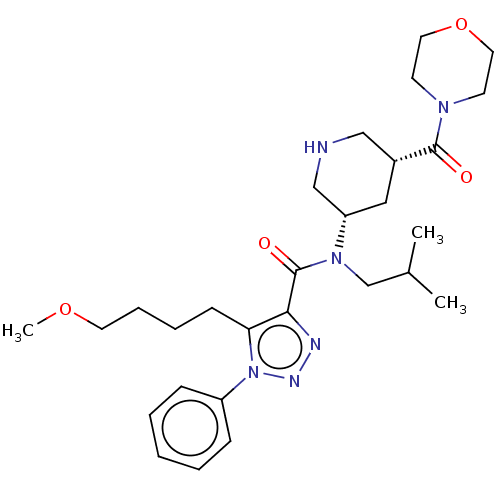 (CHEMBL4129081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273160 (IMARIKIREN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426652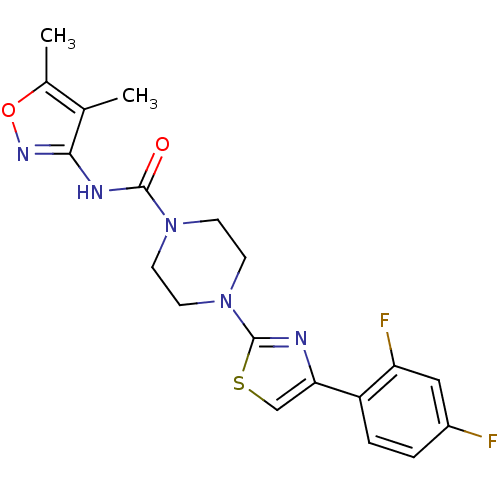 (CHEMBL2326178) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426652 (CHEMBL2326178) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195224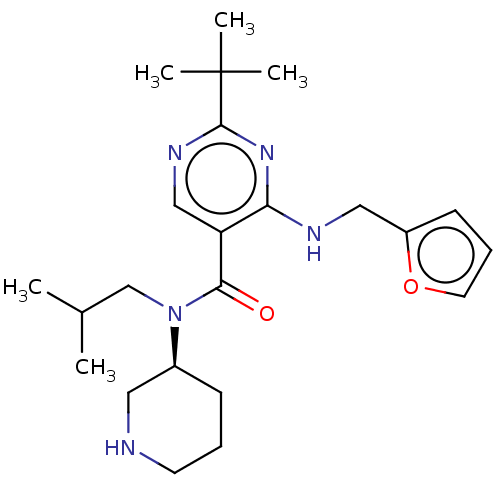 (CHEMBL3916240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195788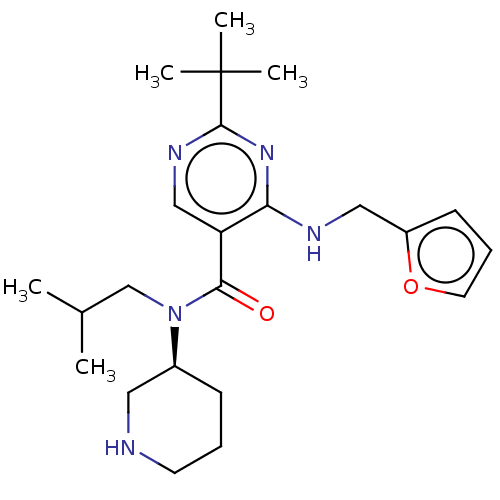 (CHEMBL3961799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273174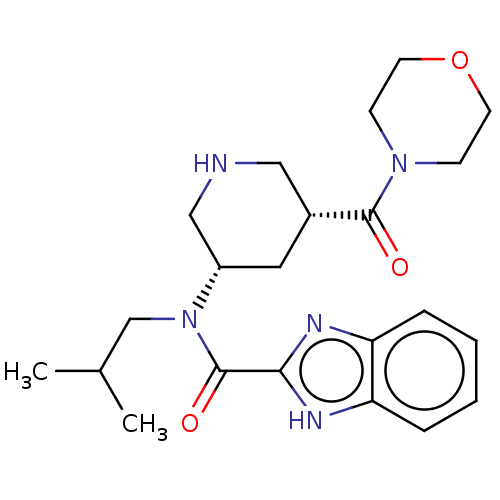 (CHEMBL4125900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273161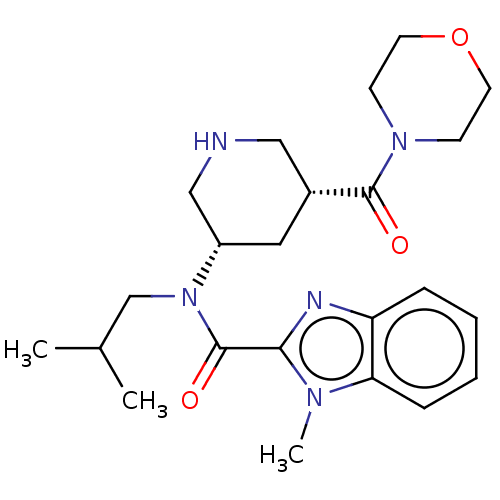 (CHEMBL4127179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273163 (CHEMBL4126136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273202 (CHEMBL4129081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273161 (CHEMBL4127179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273172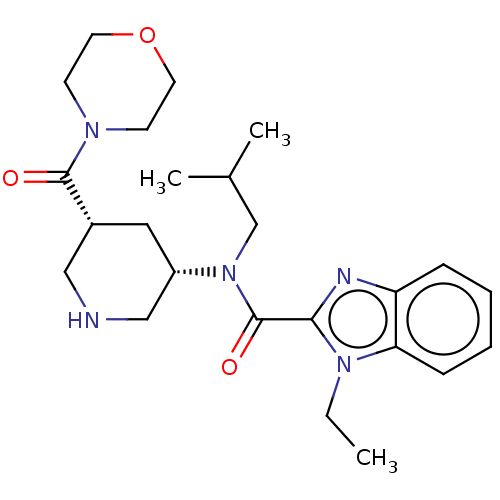 (CHEMBL4129865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426651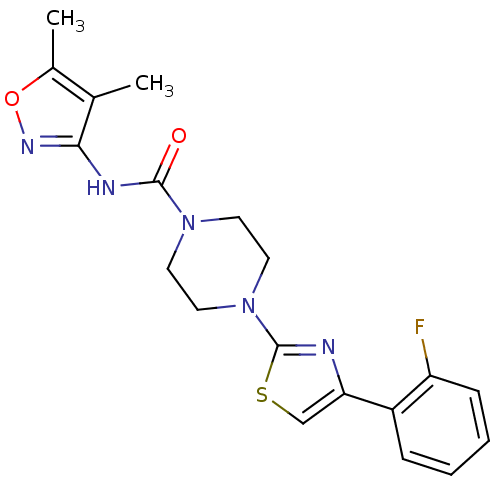 (CHEMBL2326194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426650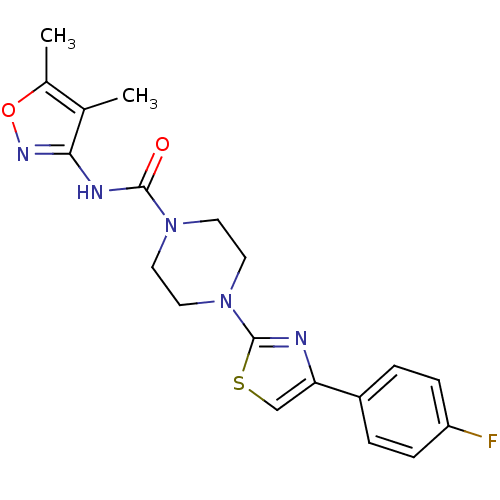 (CHEMBL2326177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273162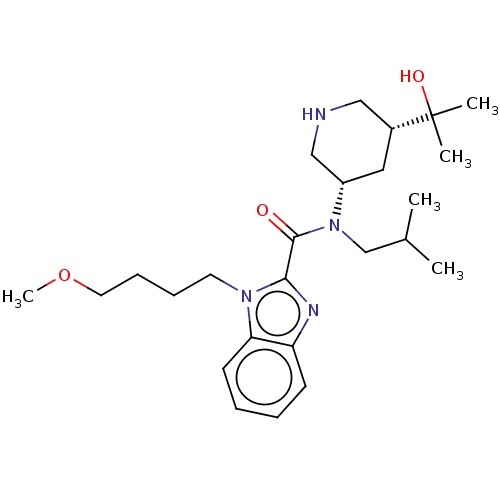 (CHEMBL4128795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426651 (CHEMBL2326194) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273174 (CHEMBL4125900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195782 (CHEMBL3958809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195782 (CHEMBL3958809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426648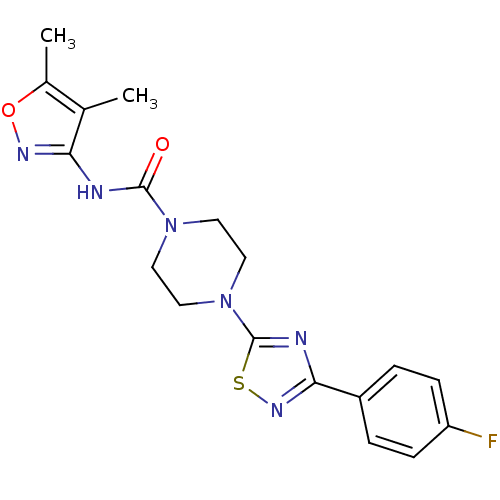 (CHEMBL2326197) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273173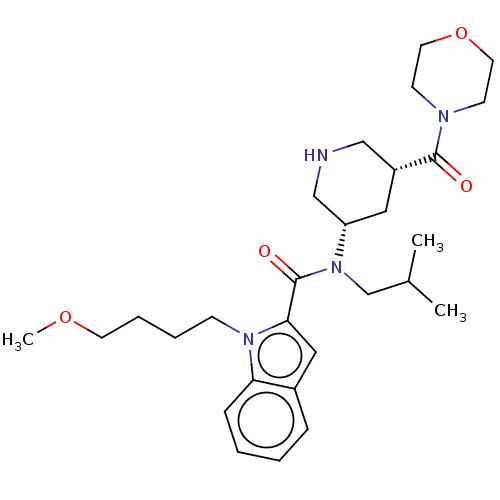 (CHEMBL4129207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273170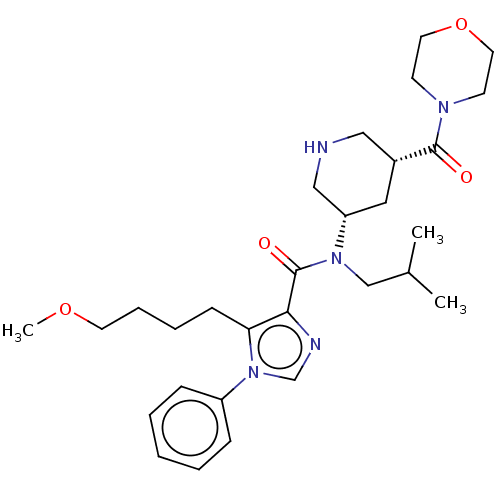 (CHEMBL4130109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273160 (IMARIKIREN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426650 (CHEMBL2326177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273172 (CHEMBL4129865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273199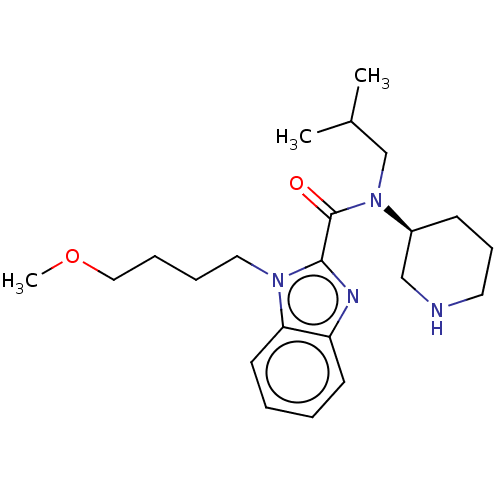 (CHEMBL4128438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426649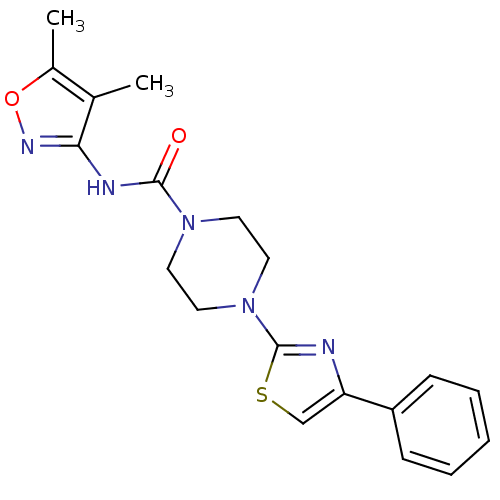 (CHEMBL2326192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426649 (CHEMBL2326192) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273202 (CHEMBL4129081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273196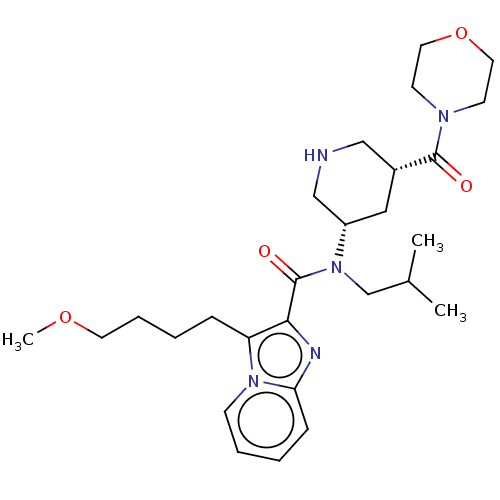 (CHEMBL4127842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273170 (CHEMBL4130109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273171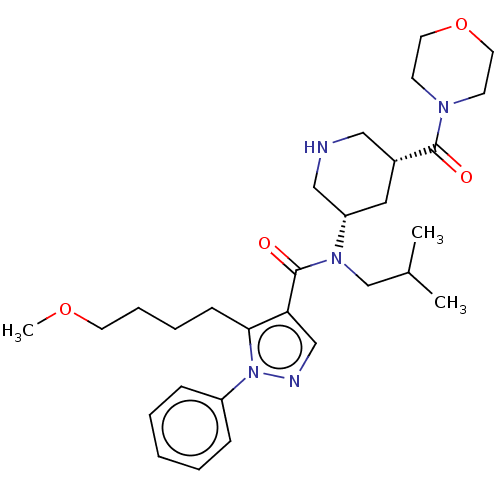 (CHEMBL4127686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50426645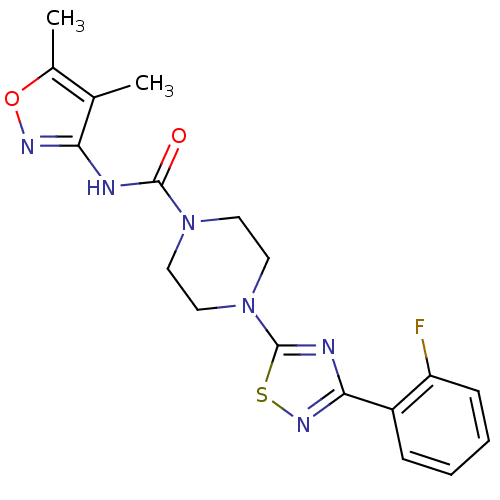 (CHEMBL2326193) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of rat FAAH after 30 mins | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273201 (CHEMBL4126453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca mulatta) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in monkey plasma | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195228 (CHEMBL3925242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195781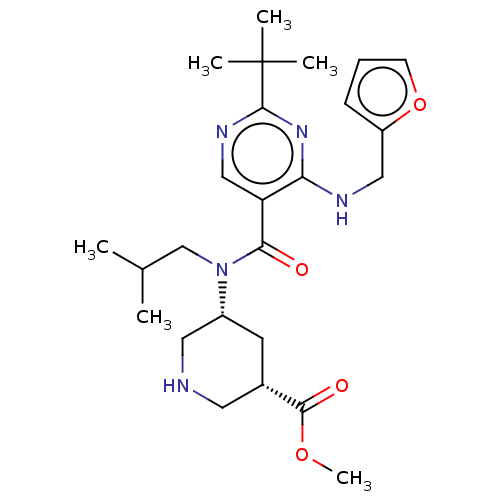 (CHEMBL3978240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human preprorenin expressed in human 293F cells using angiotensinogen as substrate preincubated for 10 mins followed by sub... | ACS Med Chem Lett 7: 933-938 (2016) Article DOI: 10.1021/acsmedchemlett.6b00251 BindingDB Entry DOI: 10.7270/Q2SF2Z3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50426647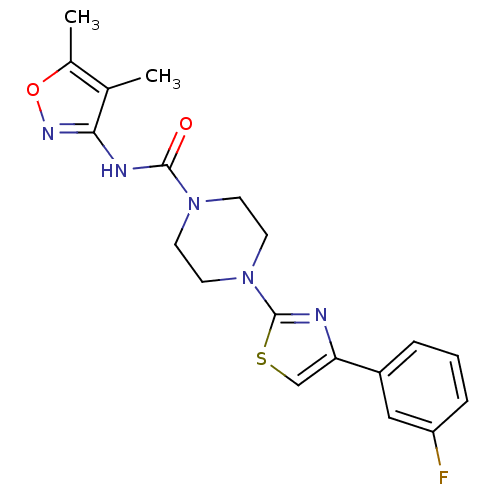 (CHEMBL2326196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting | Bioorg Med Chem 21: 28-41 (2012) Article DOI: 10.1016/j.bmc.2012.11.006 BindingDB Entry DOI: 10.7270/Q2FN17H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273170 (CHEMBL4130109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in human plasma by radioimmunoassay | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50273171 (CHEMBL4127686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using angiotensinogen as substrate pretreated for 10 mins followed by substrate addition measured after 30 mins... | Bioorg Med Chem 26: 3261-3286 (2018) Article DOI: 10.1016/j.bmc.2018.04.051 BindingDB Entry DOI: 10.7270/Q2VH5RCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |