

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096484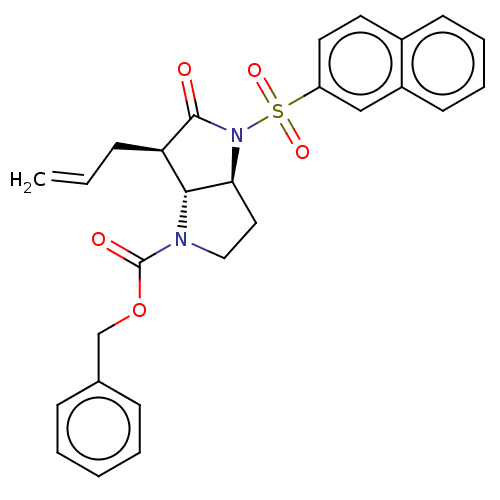 ((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997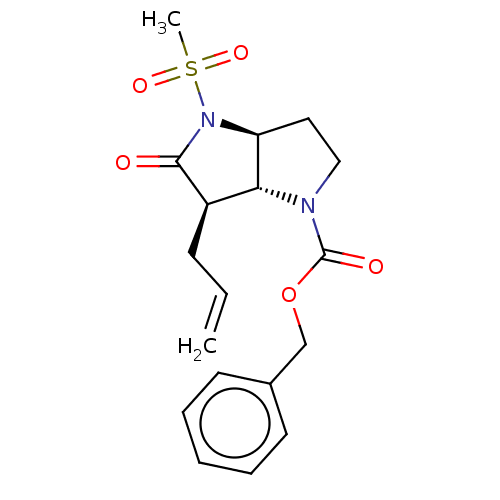 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096486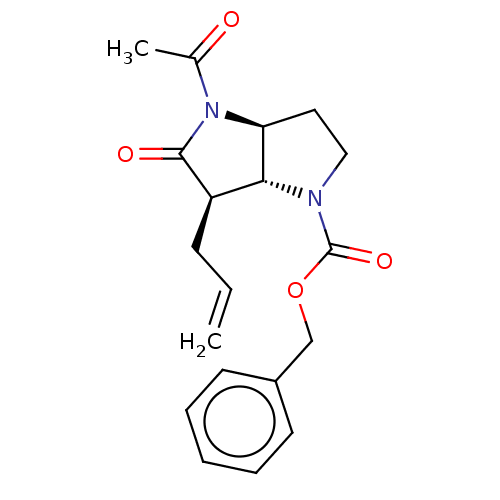 (CHEMBL2367646 | benzyl (3aS,6aR)-4-acetyl-6-allyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488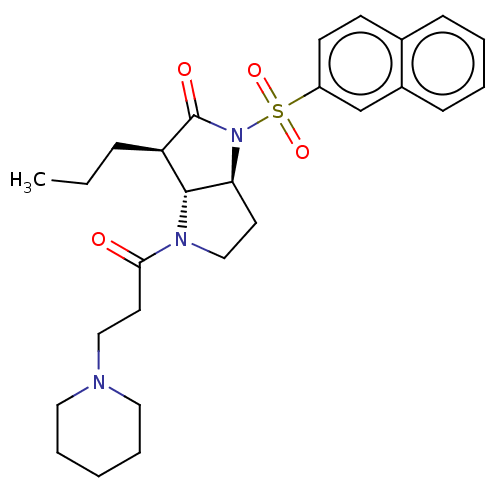 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity for dopamine D4-like receptor labelled with [3H]YM-09151-2 in retina | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096487 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061024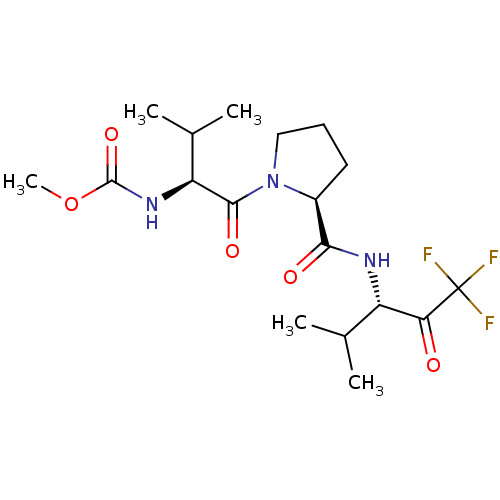 ((2-Methyl-1-{(S)-oxo-[(S)-2-((S)-3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096490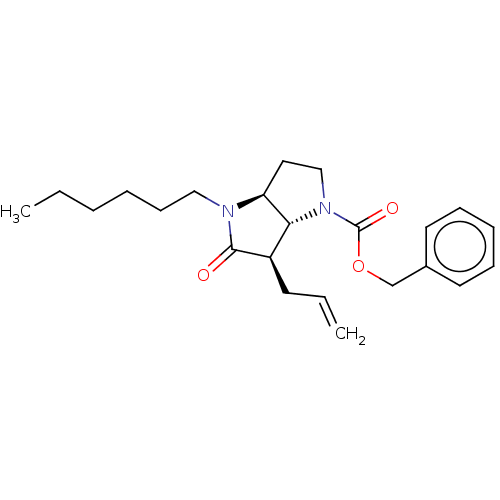 ((3aS,6R)-6-Allyl-4-hexyl-5-oxo-hexahydro-pyrrolo[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096485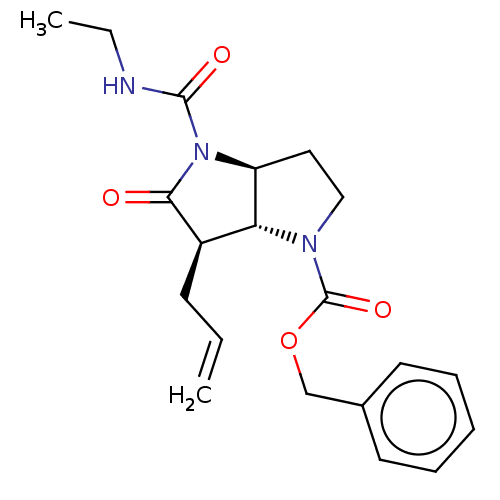 ((3aS,6R)-6-Allyl-4-ethylcarbamoyl-5-oxo-hexahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067002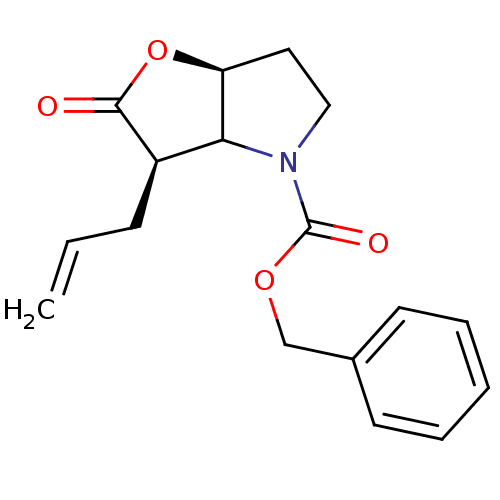 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066998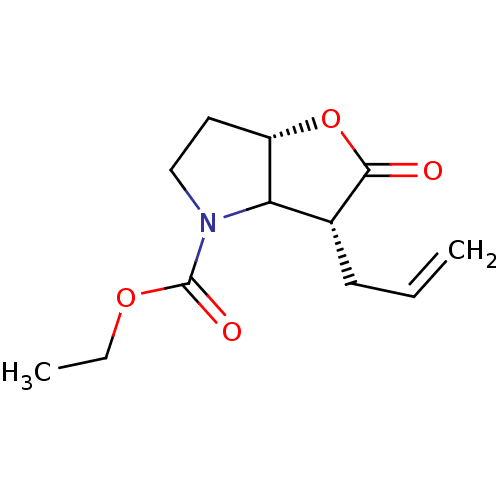 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066996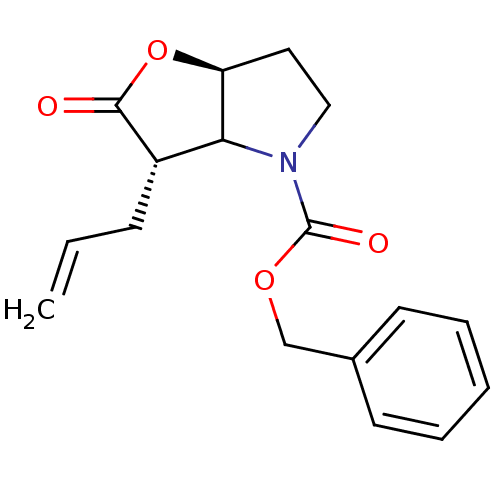 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067000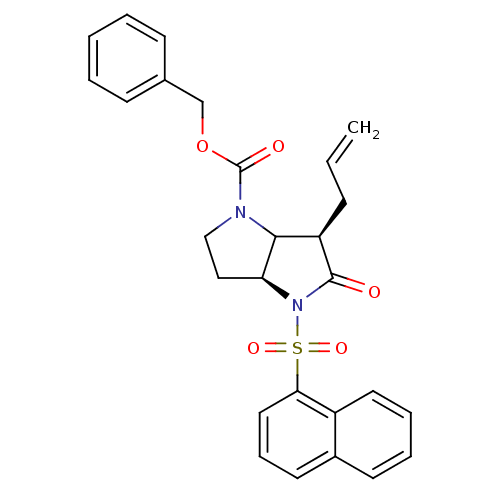 ((3aS,6R)-6-Allyl-4-(naphthalene-1-sulfonyl)-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096489 ((3aS,6R)-6-Allyl-4-formyl-5-oxo-hexahydro-pyrrolo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067002 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067001 ((3aS,6R)-6-Allyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50066998 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of cathepsin G with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066998 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066996 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50067000 ((3aS,6R)-6-Allyl-4-(naphthalene-1-sulfonyl)-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066996 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50067002 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of cathepsin G with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhition of thrombin with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066996 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhition of thrombin with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067002 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhition of thrombin with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50066996 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of cathepsin G with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of thrombin with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067000 ((3aS,6R)-6-Allyl-4-(naphthalene-1-sulfonyl)-5-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of thrombin with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50067001 ((3aS,6R)-6-Allyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067000 ((3aS,6R)-6-Allyl-4-(naphthalene-1-sulfonyl)-5-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067001 ((3aS,6R)-6-Allyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of thrombin with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50067002 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of cathepsin G with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067001 ((3aS,6R)-6-Allyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066998 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhition of thrombin with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067001 ((3aS,6R)-6-Allyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||