

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloblastin (Homo sapiens (Human)) | BDBM50263166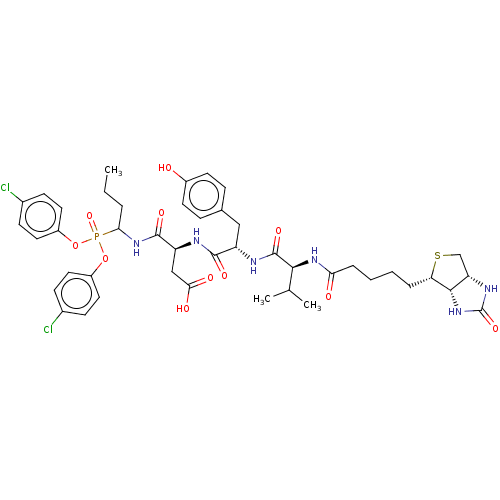 (CHEMBL4071346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France. Curated by ChEMBL | Assay Description Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay | J Med Chem 61: 1858-1870 (2018) Article DOI: 10.1021/acs.jmedchem.7b01416 BindingDB Entry DOI: 10.7270/Q25B04ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50263172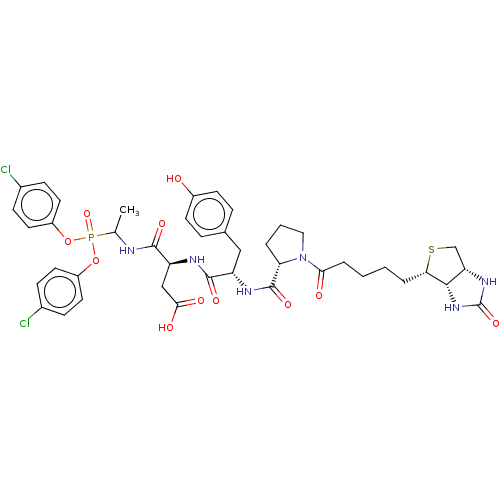 (CHEMBL4085013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France. Curated by ChEMBL | Assay Description Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay | J Med Chem 61: 1858-1870 (2018) Article DOI: 10.1021/acs.jmedchem.7b01416 BindingDB Entry DOI: 10.7270/Q25B04ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50250422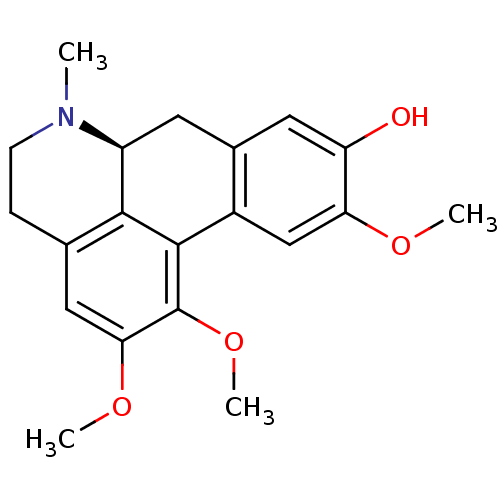 ((+)-N-methyl-laurotetanine | CHEMBL464099 | N-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in CHO cells | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028378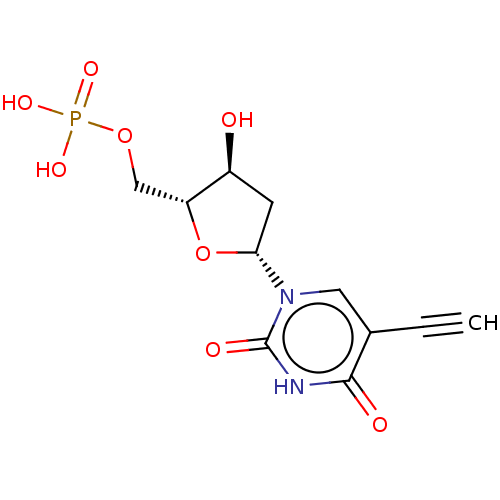 (CHEMBL3143871 | Phosphoric acid mono-[5-(5-ethynyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of dTMP synthetase from L. casei | J Med Chem 24: 1537-40 (1982) BindingDB Entry DOI: 10.7270/Q2H1311M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50263165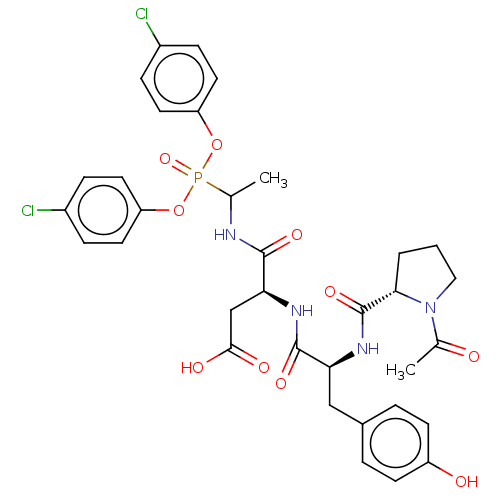 (CHEMBL4102959) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM UMR1100,"Centre d'Etude des Pathologies Respiratoires" , Université de Tours , 37032 Tours , France. Curated by ChEMBL | Assay Description Inhibition of human PR3 using ABZ-VADnVADYQ-EDDnp as substrate by FRET assay | J Med Chem 61: 1858-1870 (2018) Article DOI: 10.1021/acs.jmedchem.7b01416 BindingDB Entry DOI: 10.7270/Q25B04ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50259677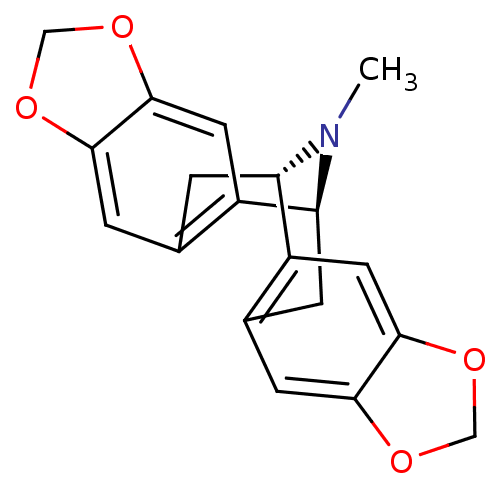 (CHEMBL481839 | escholtzine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in CHO cells | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50492323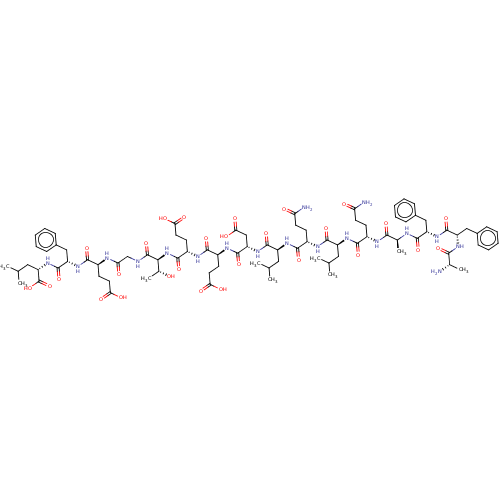 (CHEMBL2402205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of MR121-Nrf2 from human N-terminal His-tagged Kelch-DC domain of Keap1 (321 to 609) expressed in Escherichia coli BL21 (DE3) after 40 m... | Bioorg Med Chem 21: 4011-9 (2013) Article DOI: 10.1016/j.bmc.2013.04.019 BindingDB Entry DOI: 10.7270/Q2MC92ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Patatin-like phospholipase domain-containing protein 2 (Homo sapiens (Human)) | BDBM50185419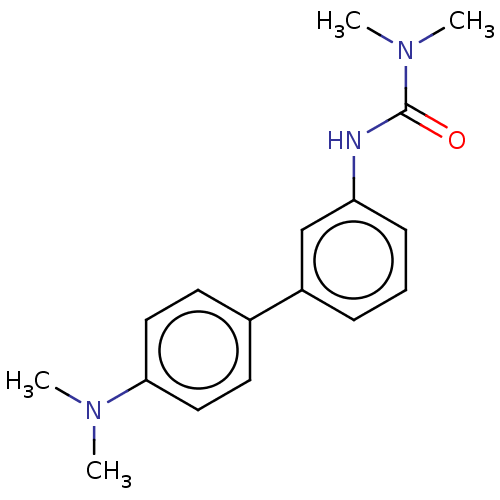 (CHEMBL3823931) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Moncton Curated by ChEMBL | Assay Description Inhibition of ATGL (unknown origin) overexpressed in Escherichia coli XL-1 cells using [9,10-3H(N)]triolein as substrate incubated for 60 mins by liq... | Eur J Med Chem 118: 290-8 (2016) Article DOI: 10.1016/j.ejmech.2016.04.021 BindingDB Entry DOI: 10.7270/Q20V8FQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50006931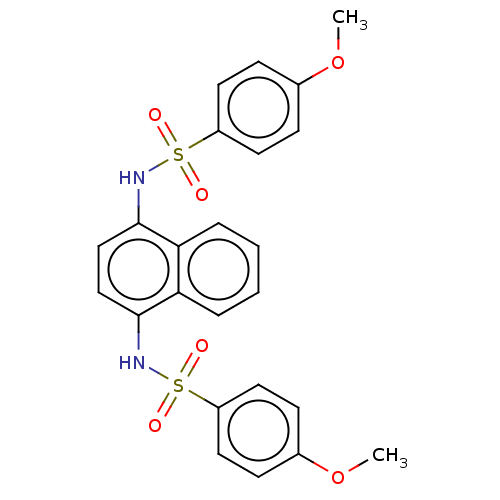 (CHEMBL2402207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of MR121-Nrf2 from human N-terminal His-tagged Kelch-DC domain of Keap1 (321 to 609) expressed in Escherichia coli BL21 (DE3) after 40 m... | Bioorg Med Chem 21: 4011-9 (2013) Article DOI: 10.1016/j.bmc.2013.04.019 BindingDB Entry DOI: 10.7270/Q2MC92ZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50259677 (CHEMBL481839 | escholtzine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50242284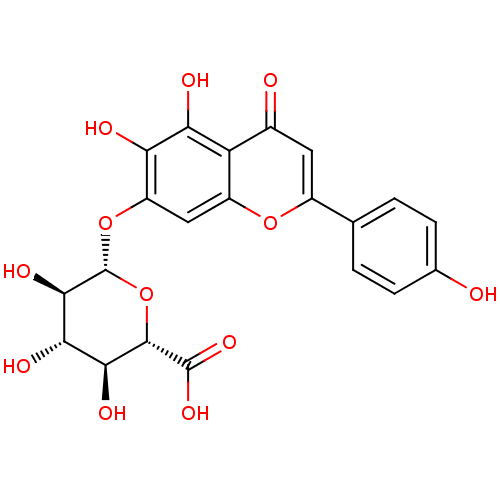 (CHEMBL487805 | scutellarin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50377937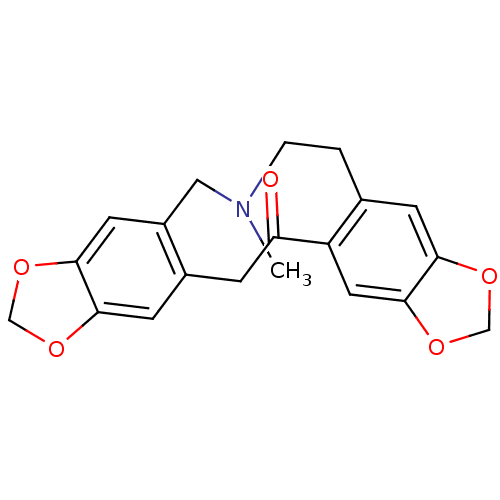 (PROTOPINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50377936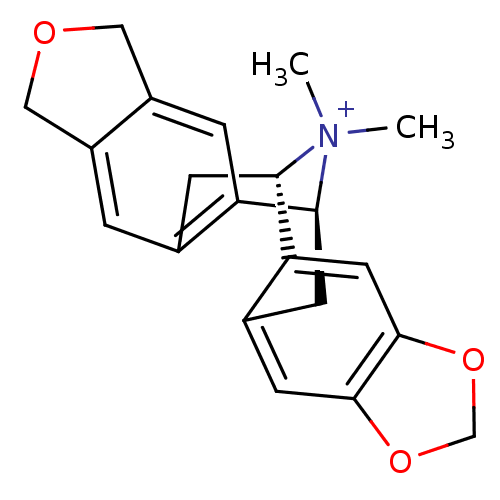 (CALIFORNIDINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50217942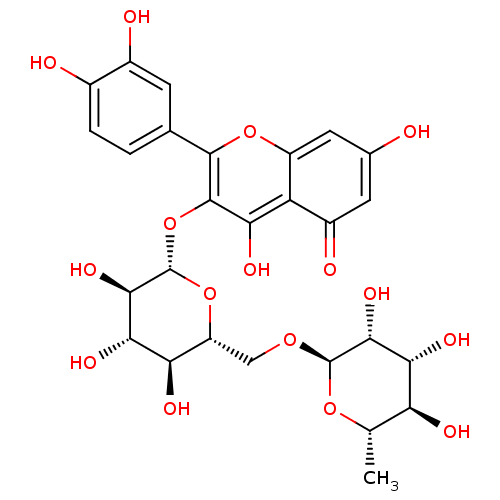 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50120421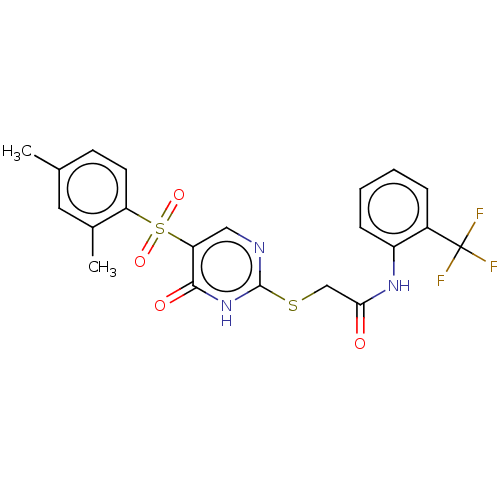 (CHEMBL2402206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc. Curated by ChEMBL | Assay Description Displacement of MR121-Nrf2 from human N-terminal His-tagged Kelch-DC domain of Keap1 (321 to 609) expressed in Escherichia coli BL21 (DE3) after 40 m... | Bioorg Med Chem 21: 4011-9 (2013) Article DOI: 10.1016/j.bmc.2013.04.019 BindingDB Entry DOI: 10.7270/Q2MC92ZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50250624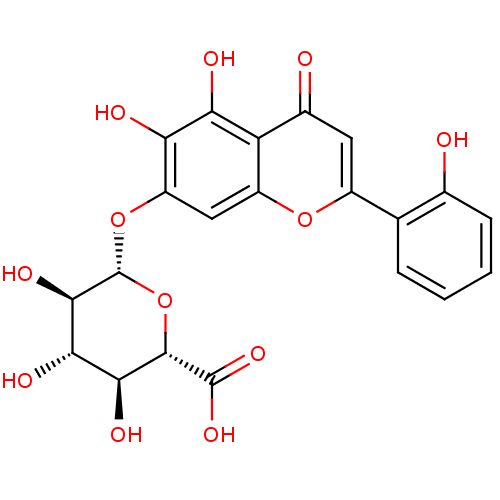 (7-glucuronyloxy-5,6,2'-trihydroxyflavone | CHEMBL5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50250625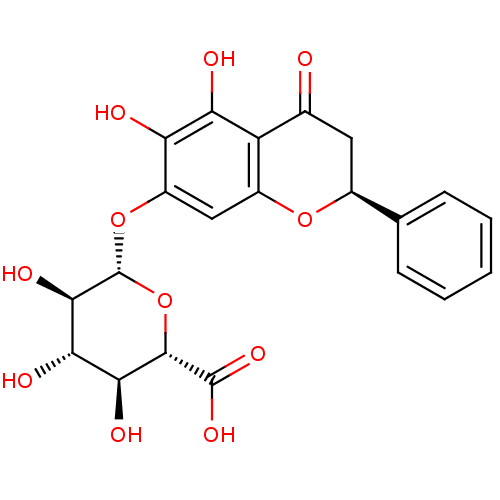 (CHEMBL467197 | dihydrobaicalin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50242173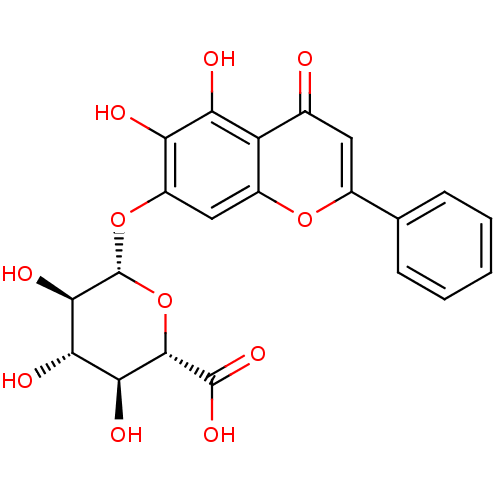 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50140257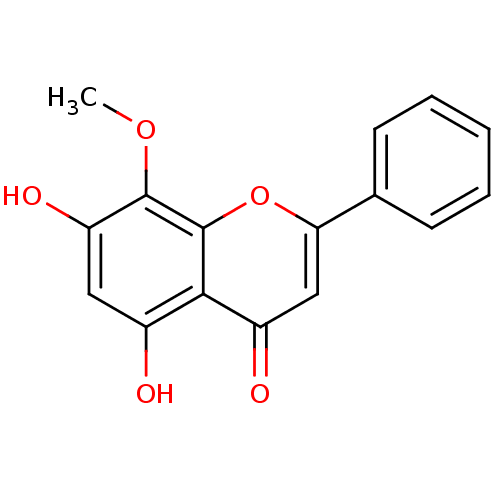 (5,7-Dihydroxy-8-methoxy-2-phenyl-chromen-4-one | 5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127518 (CHEMBL57104 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127519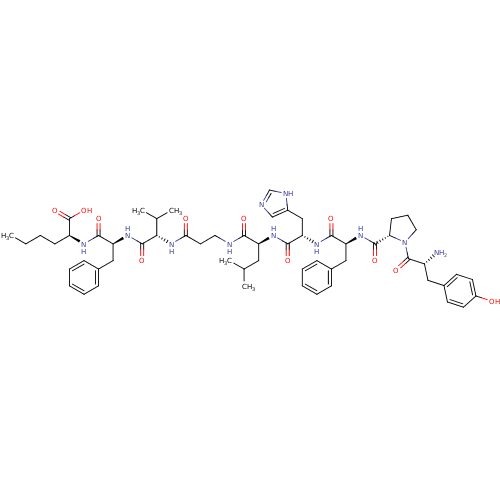 (CHEMBL262992 | [D-Tyr6-, Beta-Ala11, Phe13, Nle14]...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127520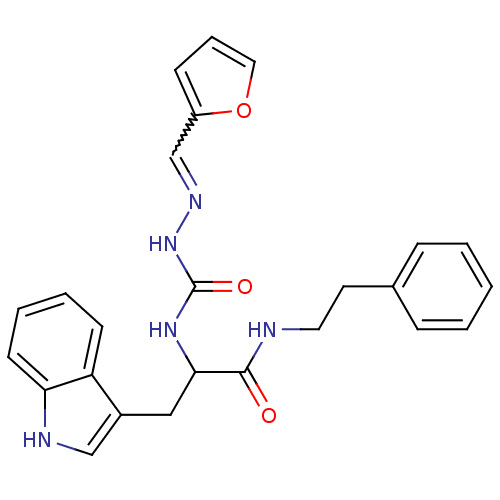 (CHEMBL57438 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-B receptor (Homo sapiens (Human)) | BDBM50127519 (CHEMBL262992 | [D-Tyr6-, Beta-Ala11, Phe13, Nle14]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against nueromedin B receptor (NMB-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (Homo sapiens (Human)) | BDBM50127522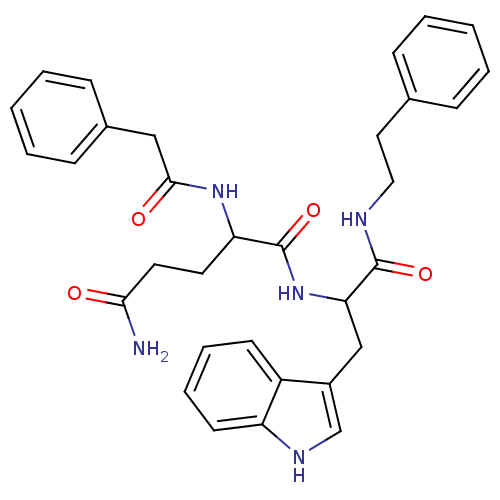 (2-Phenylacetylamino-pentanedioic acid 5-amide 1-{[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against gastrin releasing peptide receptor (GRP-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127521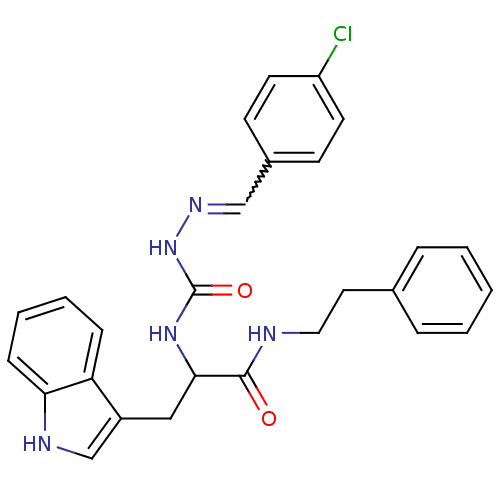 (CHEMBL57046 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127525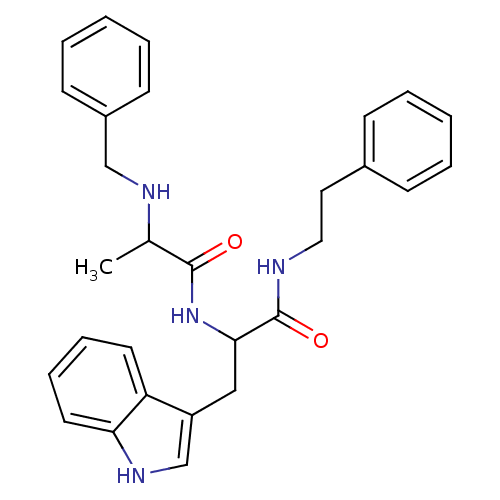 (2-Benzylamino-N-[2-(1H-indol-3-yl)-1-phenethylcarb...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127523 (3-(1H-Indol-3-yl)-N-phenethyl-2-{2-[(pyridin-3-ylm...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127524 (CHEMBL58097 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127526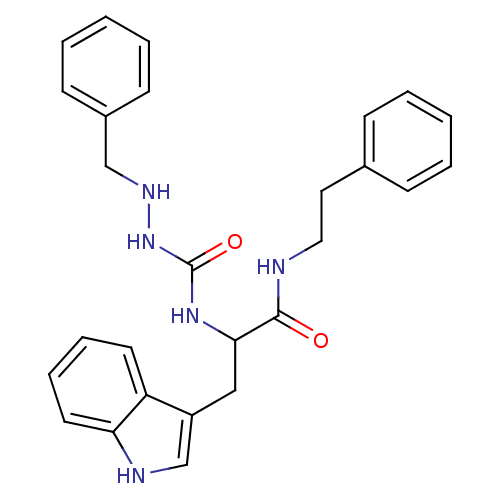 (CHEMBL56562 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127527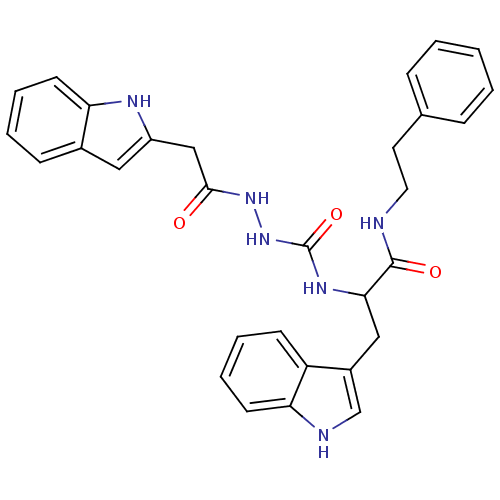 (CHEMBL294647 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127528 (3-(1H-Indol-3-yl)-N-phenethyl-2-[2-(2-1,2,3,4-tetr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (Homo sapiens (Human)) | BDBM50127519 (CHEMBL262992 | [D-Tyr6-, Beta-Ala11, Phe13, Nle14]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against gastrin releasing peptide receptor (GRP-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127529 (2-[2-(4-Chloro-benzylamino)-acetylamino]-3-(1H-ind...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127530 (2-[2-(2-Benzo[1,3]dioxol-5-yl-acetylamino)-propion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127531 (CHEMBL292227 | N-[2-(1H-Indol-3-yl)-1-phenethylcar...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-B receptor (Homo sapiens (Human)) | BDBM50127532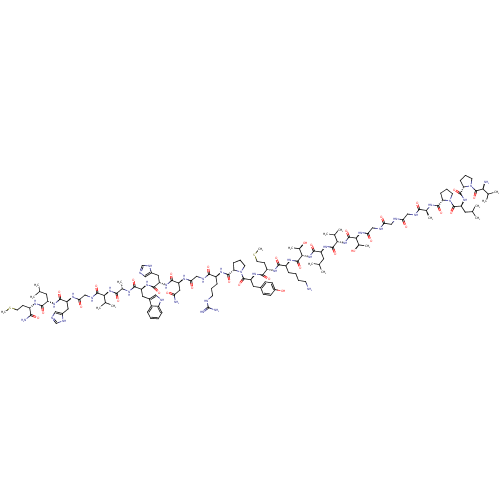 (CHEMBL438926 | Val-Pro-Leu-Pro-Ala-Gly-Gly-Gly-Thr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against nueromedin B receptor (NMB-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127532 (CHEMBL438926 | Val-Pro-Leu-Pro-Ala-Gly-Gly-Gly-Thr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127534 (CHEMBL56626 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127533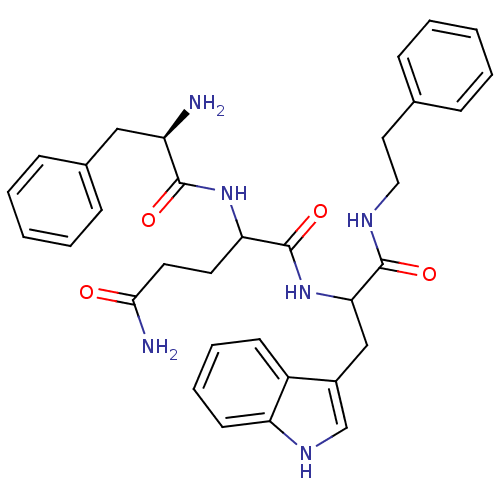 (2-(2-Amino-3-phenyl-propionylamino)-pentanedioic a...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-B receptor (Homo sapiens (Human)) | BDBM50127522 (2-Phenylacetylamino-pentanedioic acid 5-amide 1-{[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against nueromedin B receptor (NMB-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127522 (2-Phenylacetylamino-pentanedioic acid 5-amide 1-{[...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127535 (2-[2-(4-Chloro-phenyl)-acetylamino]-pentanedioic a...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127536 (2-[2-(4-Benzyl-piperidin-1-yl)-acetylamino]-3-(1H-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127538 (CHEMBL291549 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127540 (CHEMBL175651 | peptidomimetic) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127537 (3-(1H-Indol-3-yl)-2-[2-(2-1H-indol-2-yl-acetylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50127539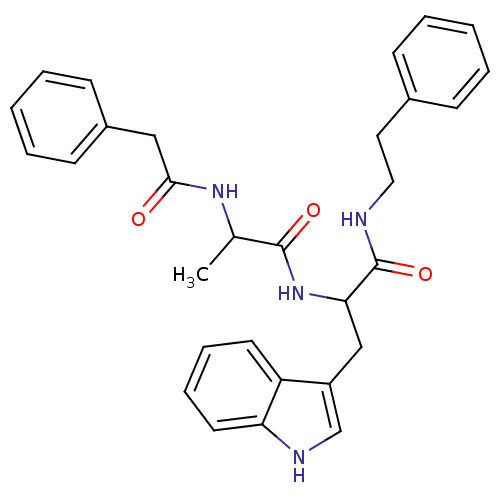 (3-(1H-Indol-3-yl)-N-phenethyl-2-(2-phenylacetylami...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against human bombesin receptor 3 (BRS-3) in CHO cells by using FLIPR assay. | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (Homo sapiens (Human)) | BDBM50071745 (CHEMBL403317 | Compound NMB | Gly-Asn-Leu-Trp-Ala-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Effective concentration required against gastrin releasing peptide receptor (GRP-R) in CHO cells by using FLIPR assay | J Med Chem 46: 1918-30 (2003) Article DOI: 10.1021/jm0210921 BindingDB Entry DOI: 10.7270/Q29S1QDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |