

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011503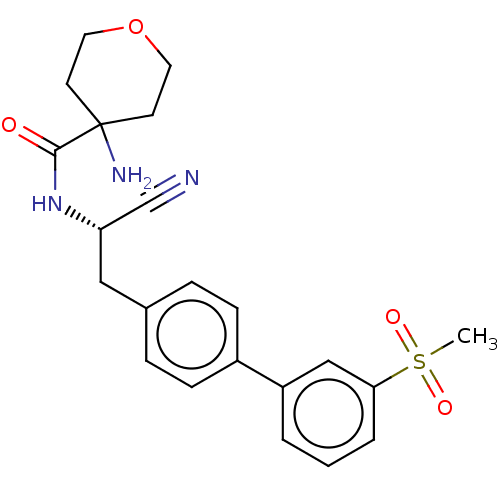 (CHEMBL3261927) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011502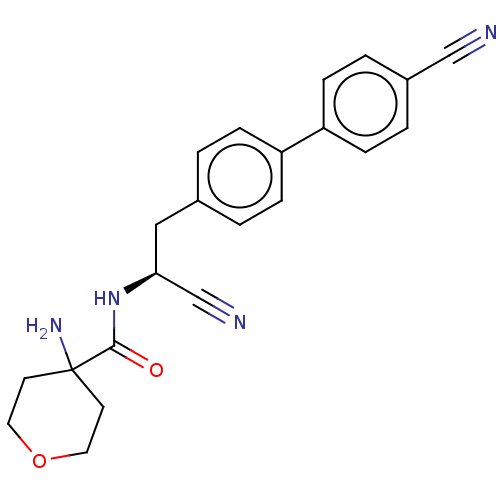 (CHEMBL3261926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011506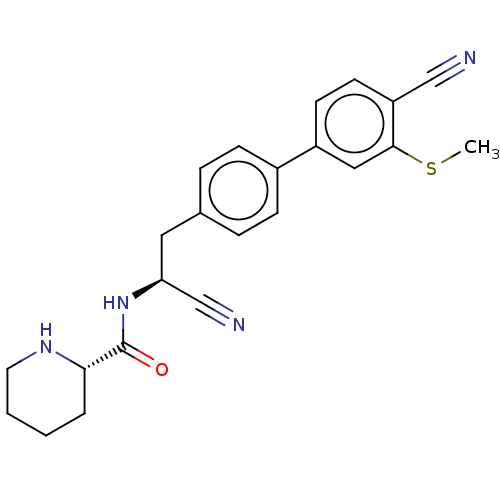 (CHEMBL3261920) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50186088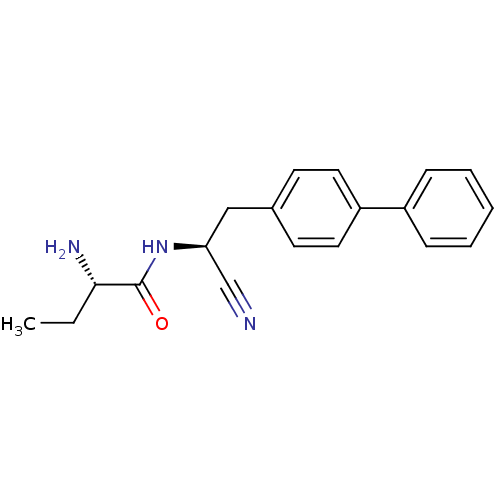 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011501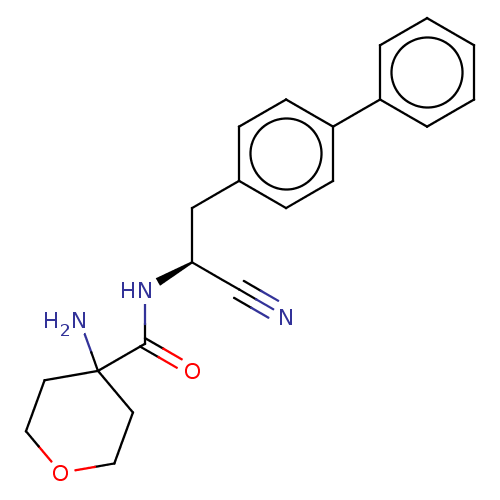 (CHEMBL3261925) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011500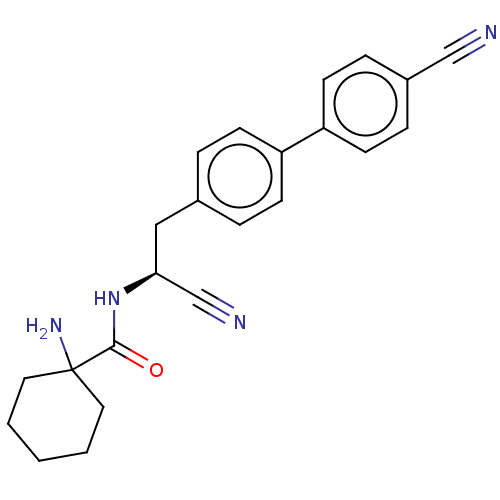 (CHEMBL3261924) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011500 (CHEMBL3261924) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011506 (CHEMBL3261920) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011509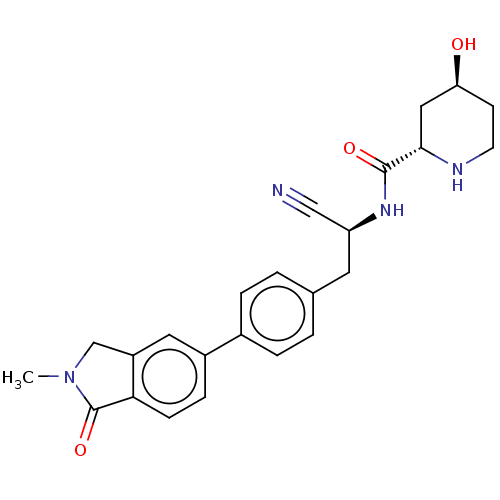 (CHEMBL3261923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011508 (CHEMBL3261922) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011502 (CHEMBL3261926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011504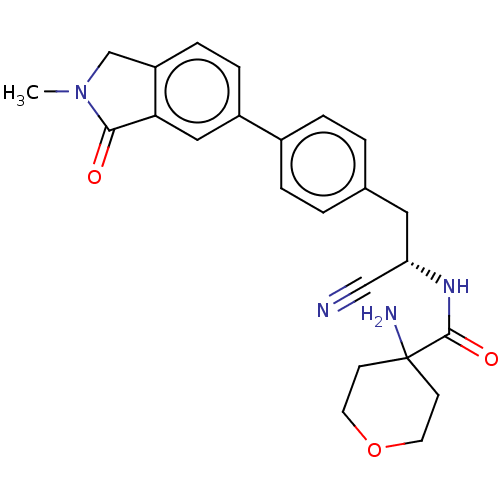 (CHEMBL3261928) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011507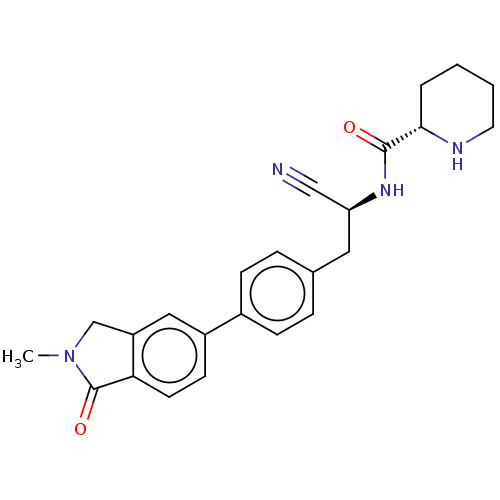 (CHEMBL3261921) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011503 (CHEMBL3261927) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011505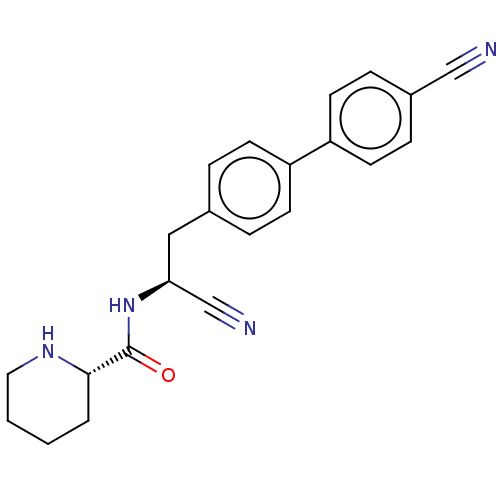 (CHEMBL3261919) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10007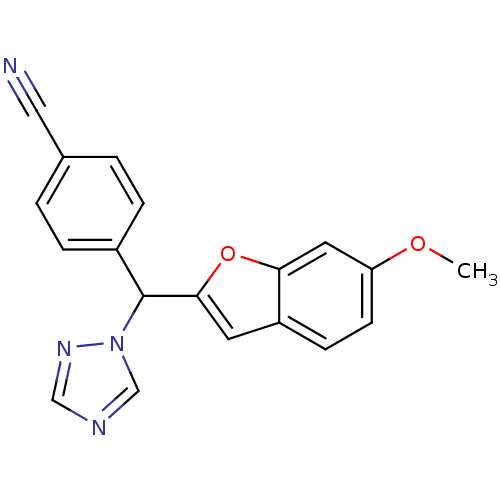 (4-[(6-methoxy-1-benzofuran-2-yl)(1H-1,2,4-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011508 (CHEMBL3261922) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10013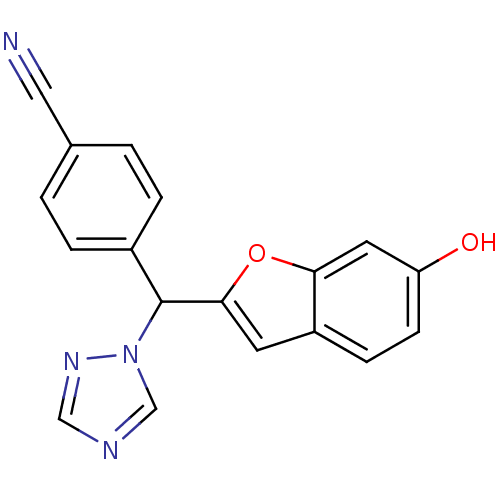 (4-[(6-Hydroxybenzofuran-2-yl)-[1,2,4]triazol-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011507 (CHEMBL3261921) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011501 (CHEMBL3261925) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011505 (CHEMBL3261919) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011509 (CHEMBL3261923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10005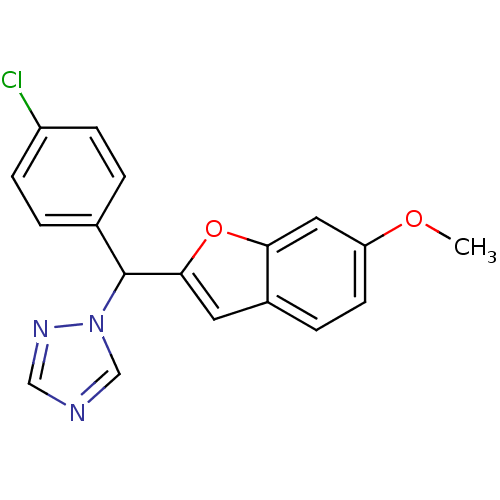 (1-[(4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10000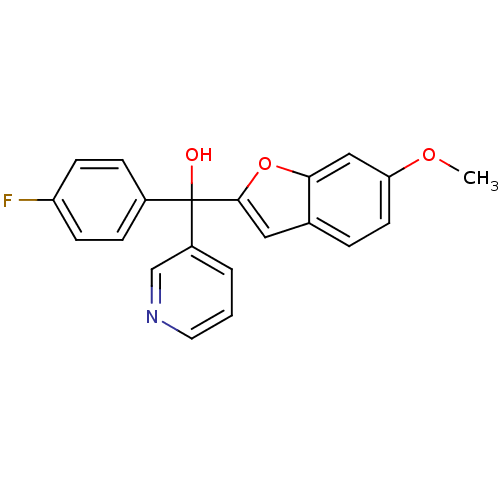 ((4-fluorophenyl)(6-methoxy-1-benzofuran-2-yl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10004 (1-[(4-fluorophenyl)(6-methoxy-1-benzofuran-2-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10001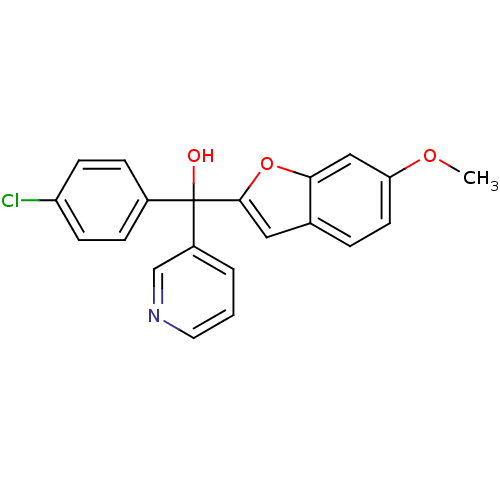 ((4-chlorophenyl)(6-methoxy-1-benzofuran-2-yl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011504 (CHEMBL3261928) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10014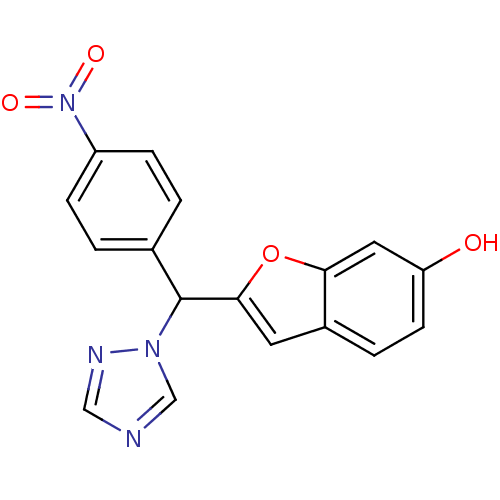 (2-[(4-Nitrophenyl)-[1,2,4]triazol-1-ylmethyl]benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50186088 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10009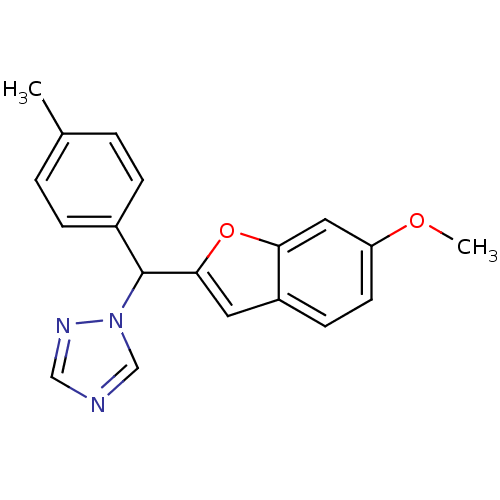 (1-[(6-Methoxybenzofuran-2-yl)-p-tolylmethyl]-1H-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011510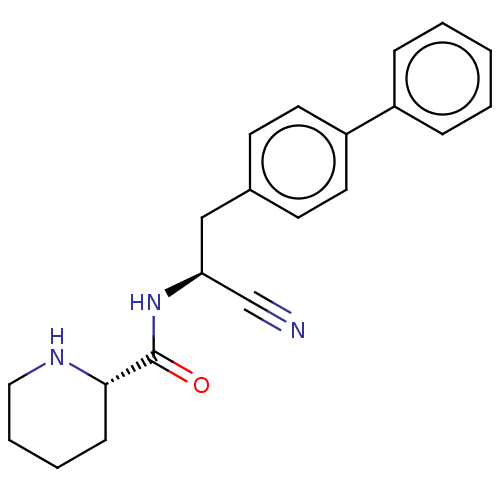 (CHEMBL3261918) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin C using H-Gly-Arg-AMC as substrate preincubated for 30 mins before substrate addition measured after 60 min... | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10003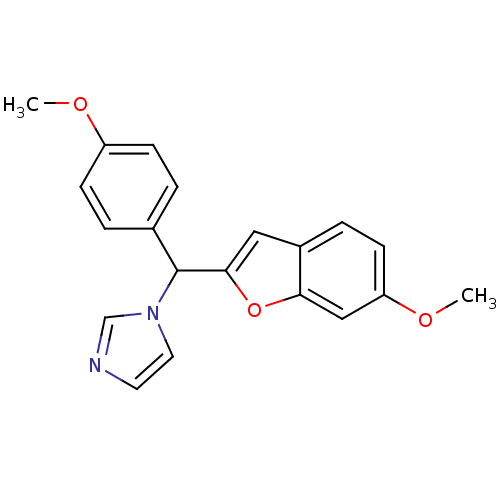 (1-[(6-Methoxybenzofuran-2-yl)-(4-methoxyphenyl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10006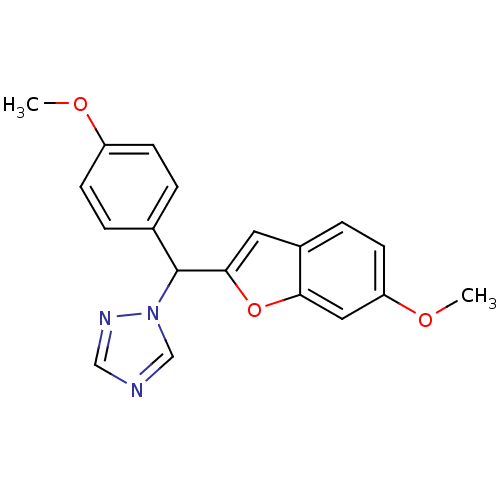 (1-[(6-Methoxybenzofuran-2-yl)-(4-methoxyphenyl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10010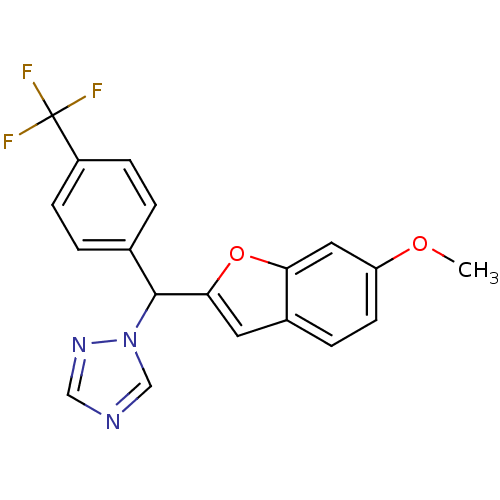 (1-[(6-Methoxybenzofuran-2-yl)-(4-trifluoromethylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50011510 (CHEMBL3261918) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin C in human THP1 cells assessed as inhibition of H-Gly-Phe-AFC cleavage by fluorescence assay | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10002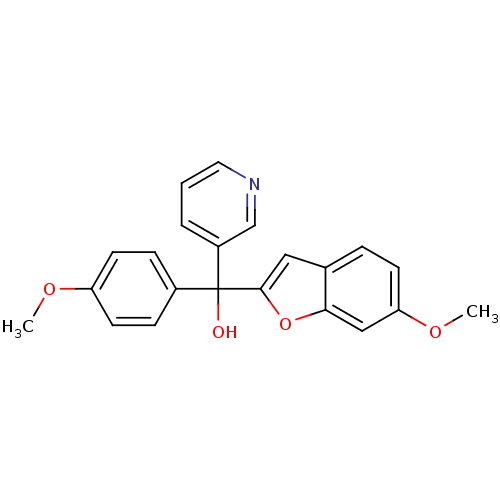 ((6-Methoxybenzofuran-2-yl)-(4-methoxyphenyl)-3-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50011507 (CHEMBL3261921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Cathepsin K (unknown origin) | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50011506 (CHEMBL3261920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Cathepsin K (unknown origin) | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50308952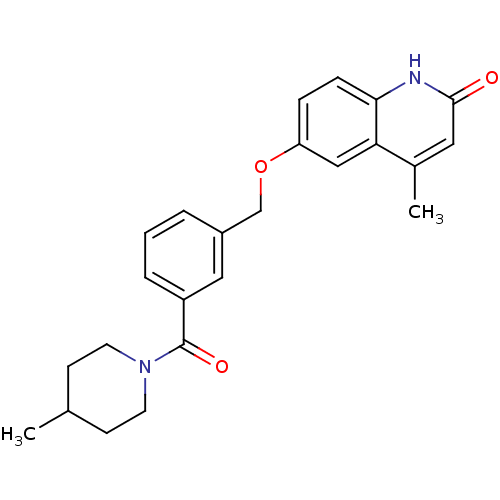 (4-Methyl-6-({3-[(4-methylpiperidino)carbonyl]-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3A by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10008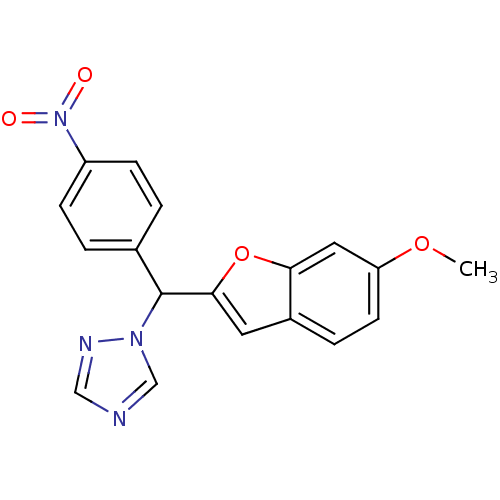 (1-[(6-Methoxybenzofuran-2-yl)-(4-nitrophenyl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015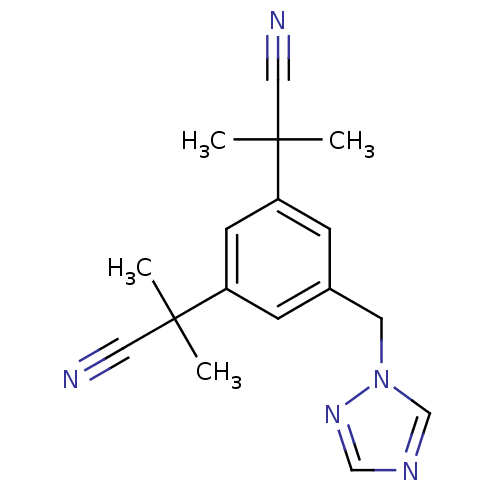 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 37 |
Cardiff University | Assay Description The classical 3H2O assay was used to measure the effect of the inhibitor compounds on aromatase activity using human placental microsomes. | J Enzyme Inhib Med Chem 20: 135-41 (2005) Article DOI: 10.1080/14756360400015256 BindingDB Entry DOI: 10.7270/Q2B56H9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50308954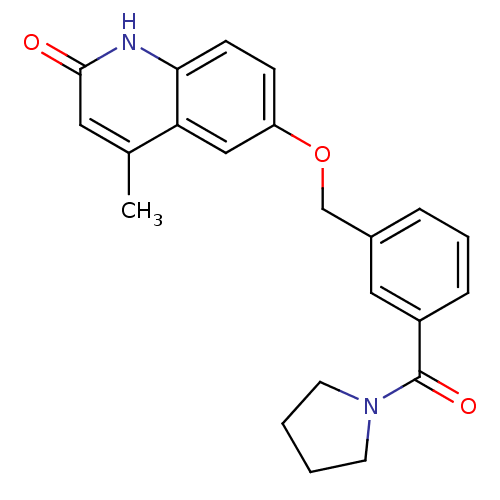 (4-Methyl-6-{[3-(tetrahydro-1H-1-pyrrolylcarbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3A by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50308951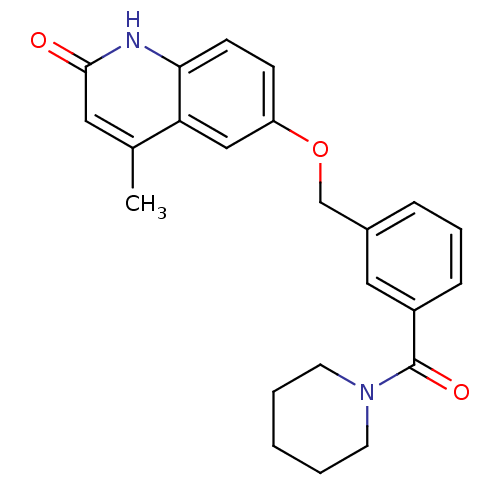 (4-Methyl-6-{[3-(piperidinocarbonyl)benzyl]oxy}-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3A by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50308949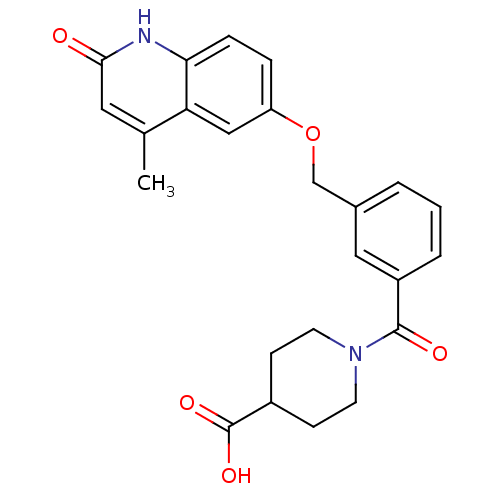 (1-(3-{[(4-Methyl-2-oxo-1,2-dihydro-6-quinolinyl)ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3A by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM50308950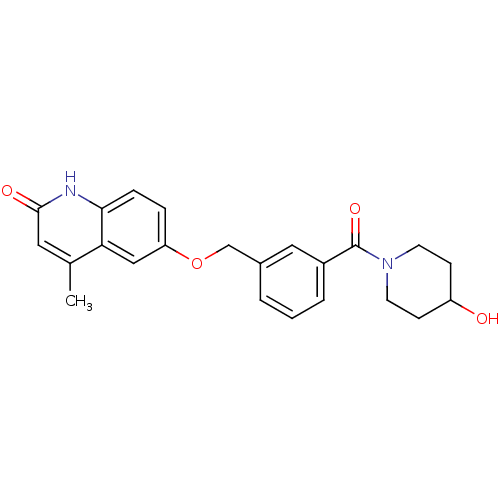 (6-({3-[(4-Hydroxypiperidino)carbonyl]benzyl}oxy)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3B by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50011505 (CHEMBL3261919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Cathepsin K (unknown origin) | J Med Chem 57: 2357-67 (2014) Article DOI: 10.1021/jm401705g BindingDB Entry DOI: 10.7270/Q20866VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM50308948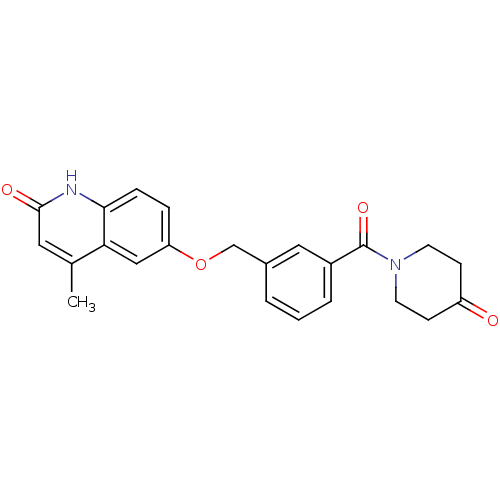 (4-Methyl-6-(3-[(4-oxopiperidino)carbonyl]benzyloxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3B by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM50308954 (4-Methyl-6-{[3-(tetrahydro-1H-1-pyrrolylcarbonyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of human PDE3B by fluorescence microplate reader | Bioorg Med Chem 18: 855-62 (2010) Article DOI: 10.1016/j.bmc.2009.11.044 BindingDB Entry DOI: 10.7270/Q2Q81F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10011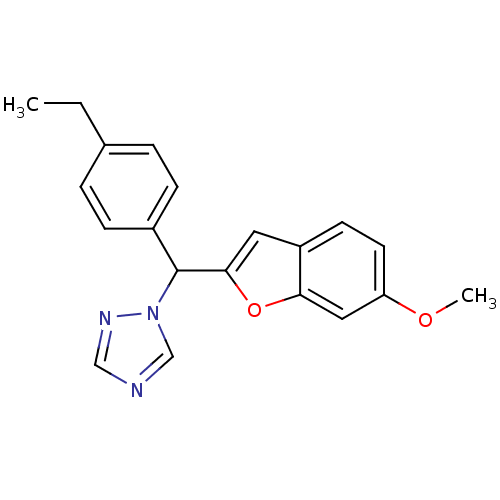 (1-[(4-ethylphenyl)(6-methoxy-1-benzofuran-2-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Cardiff University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1,2,6,7-3H ]androstenedione / androstenedione during aromatization. Afte... | J Med Chem 49: 1016-22 (2006) Article DOI: 10.1021/jm0508282 BindingDB Entry DOI: 10.7270/Q2NK3C76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |