

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Mus musculus) | BDBM50441511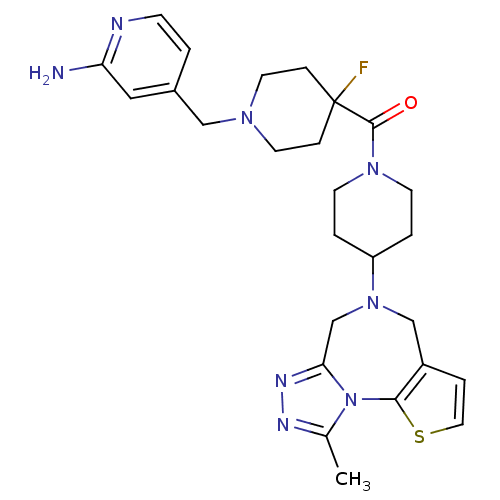 (CHEMBL2436620) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441503 (CHEMBL2436628) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441509 (CHEMBL2436622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441505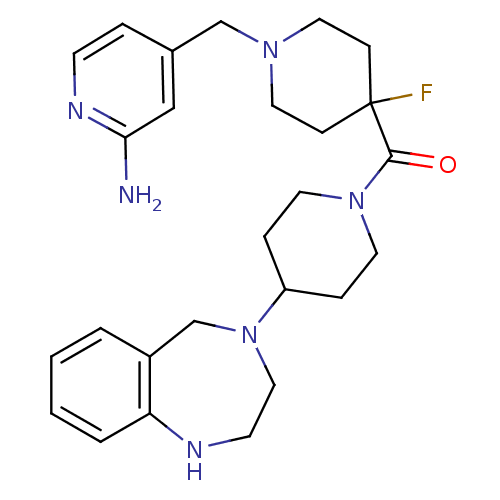 (CHEMBL2436626) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112009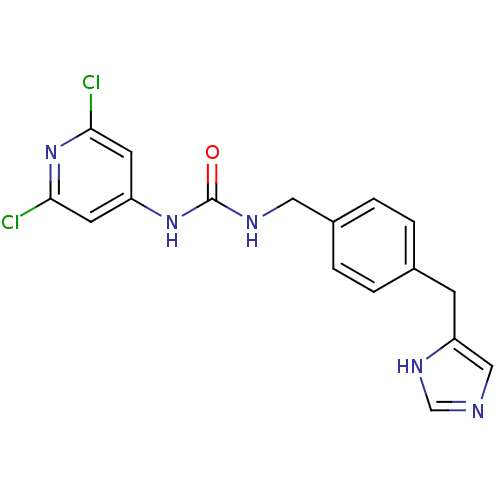 (1-(2,6-Dichloro-pyridin-4-yl)-3-[4-(1H-imidazol-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441506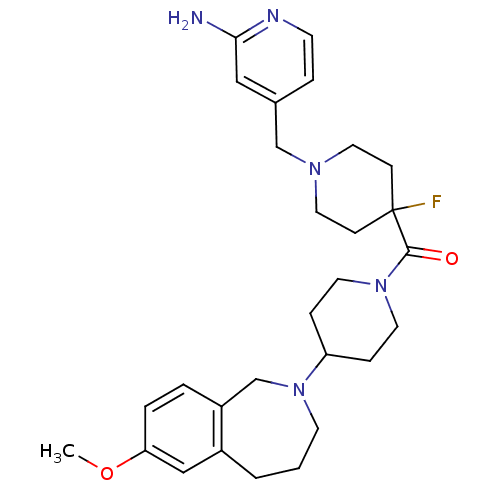 (CHEMBL2436625) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441514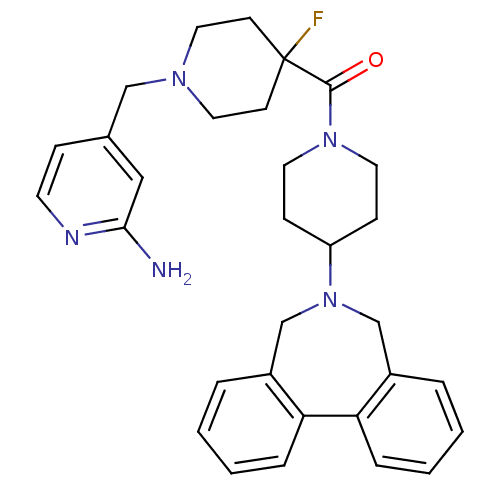 (CHEMBL2436633) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441502 (CHEMBL2436629) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441504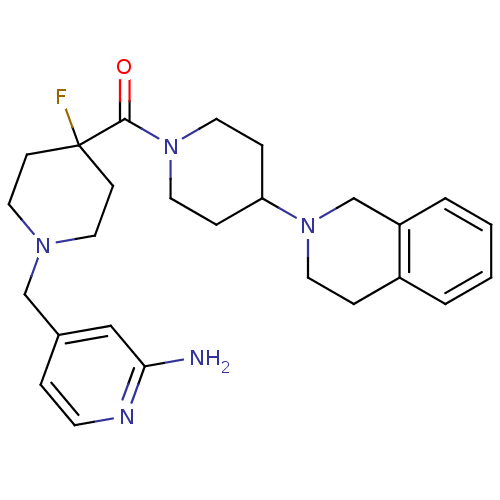 (CHEMBL2436627) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM86188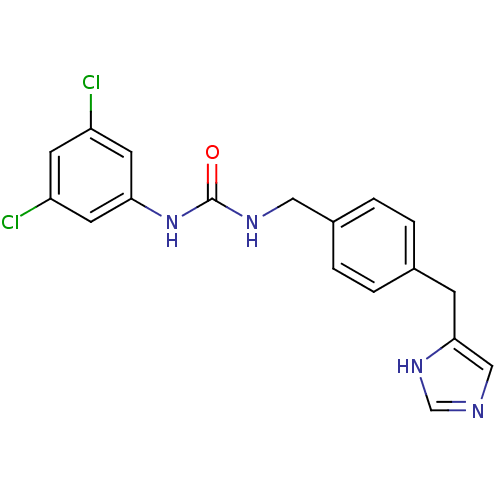 (CHEMBL366977 | SCH 79687) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441507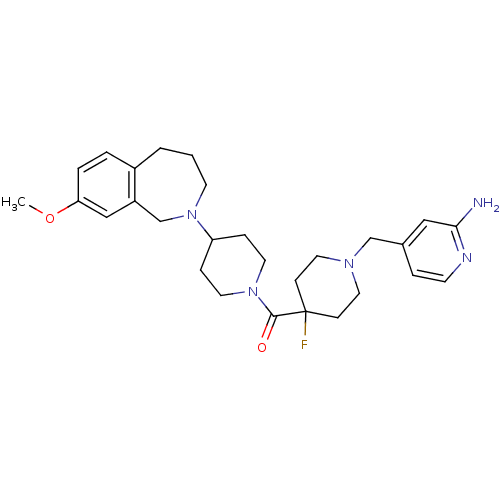 (CHEMBL2436624) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112025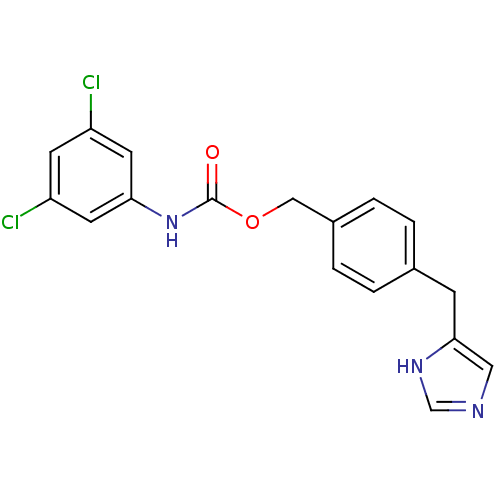 ((3,5-Dichloro-phenyl)-carbamic acid 4-(1H-imidazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112024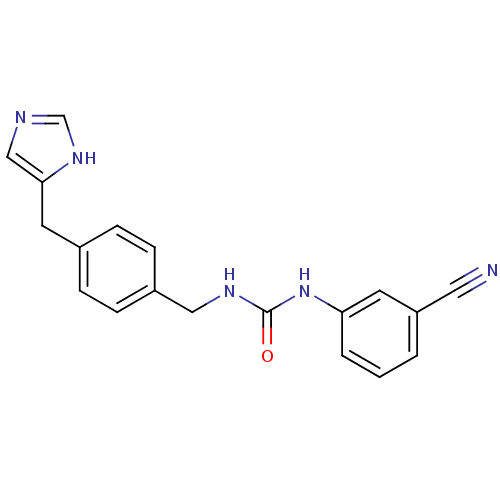 (1-(3-Cyano-phenyl)-3-[4-(1H-imidazol-4-ylmethyl)-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112012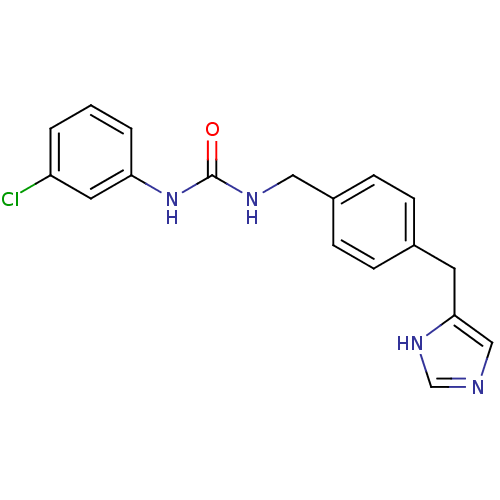 (1-(3-Chloro-phenyl)-3-[4-(1H-imidazol-4-ylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441501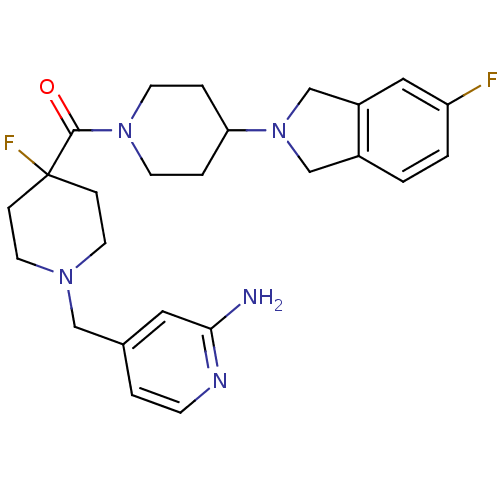 (CHEMBL2436630) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112021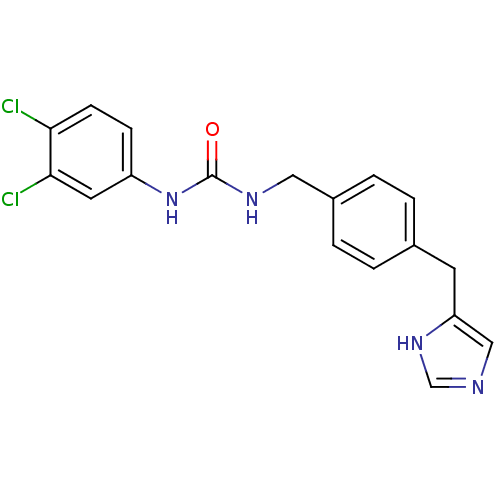 (1-(3,4-Dichloro-phenyl)-3-[4-(1H-imidazol-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441512 (CHEMBL2436617) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112011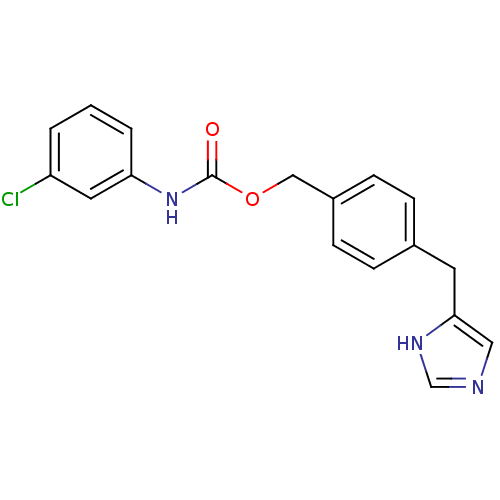 ((3-Chloro-phenyl)-carbamic acid 4-(1H-imidazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112020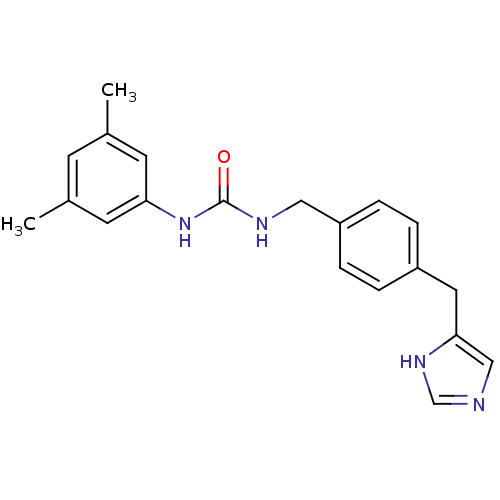 (1-(3,5-Dimethyl-phenyl)-3-[4-(1H-imidazol-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441517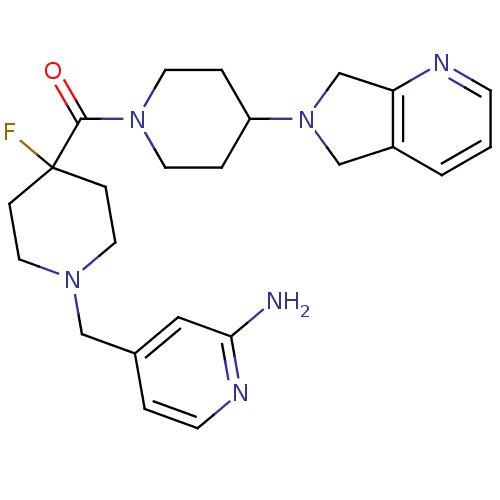 (CHEMBL2436631) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50071492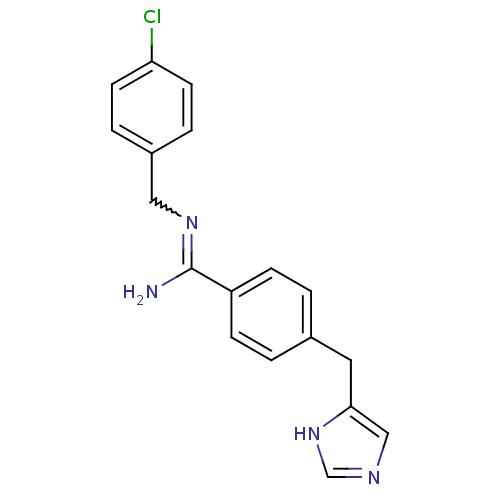 (CHEMBL312341 | N-(4-Chloro-benzyl)-4-(1H-imidazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112028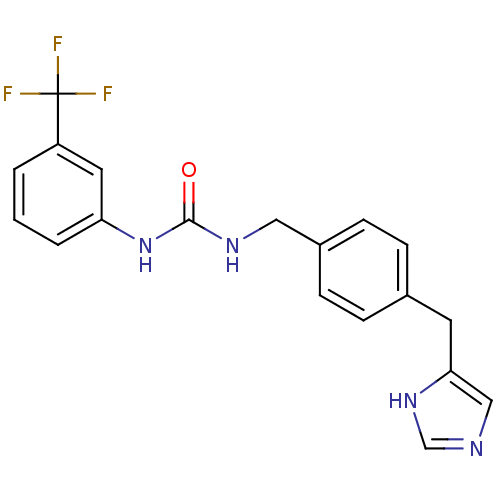 (1-[4-(1H-Imidazol-4-ylmethyl)-benzyl]-3-(3-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112013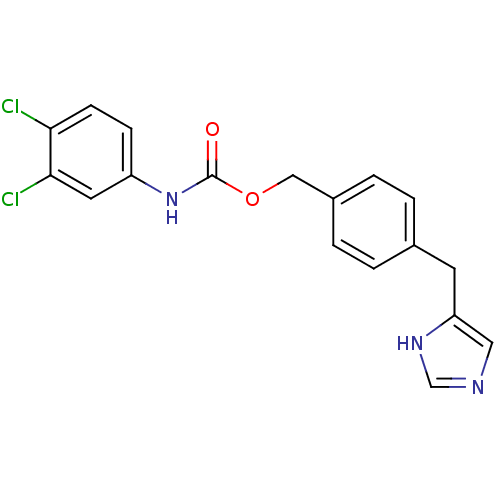 ((3,4-Dichloro-phenyl)-carbamic acid 4-(1H-imidazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112010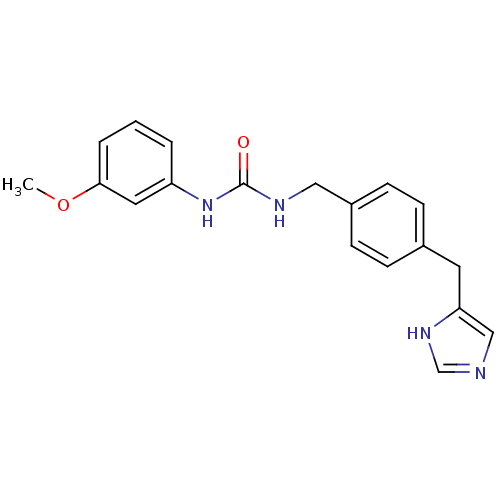 (1-[4-(1H-Imidazol-4-ylmethyl)-benzyl]-3-(3-methoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112019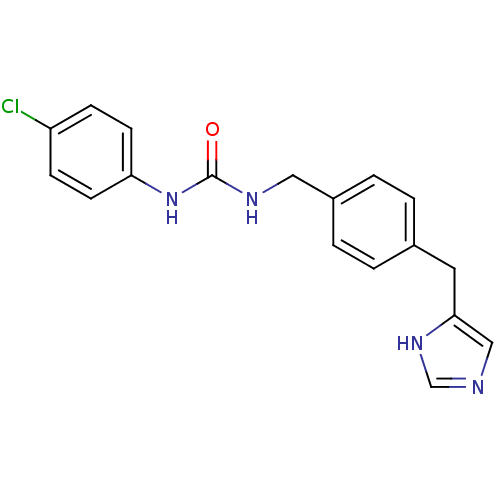 (1-(4-Chloro-phenyl)-3-[4-(1H-imidazol-4-ylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112017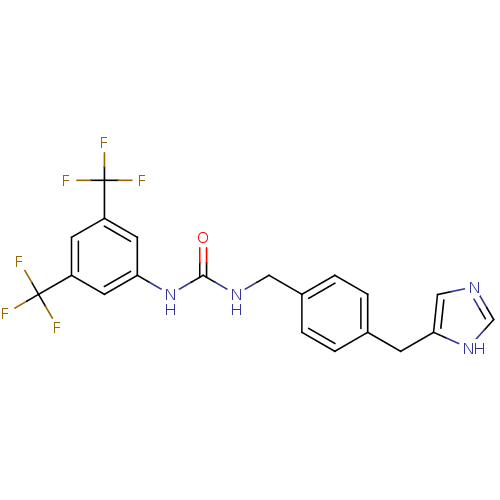 (1-(3,5-Bis-trifluoromethyl-phenyl)-3-[4-(1H-imidaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112016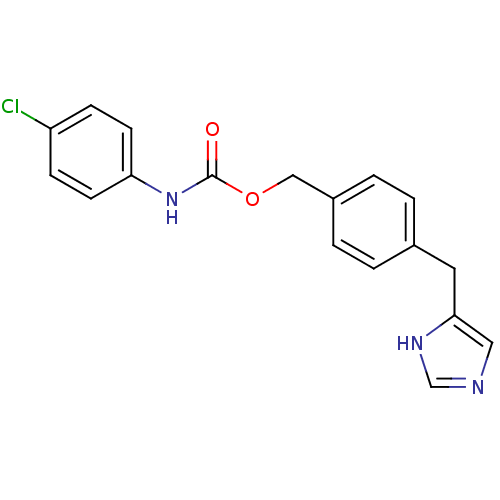 ((4-Chloro-phenyl)-carbamic acid 4-(1H-imidazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441515 (CHEMBL2436619) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112008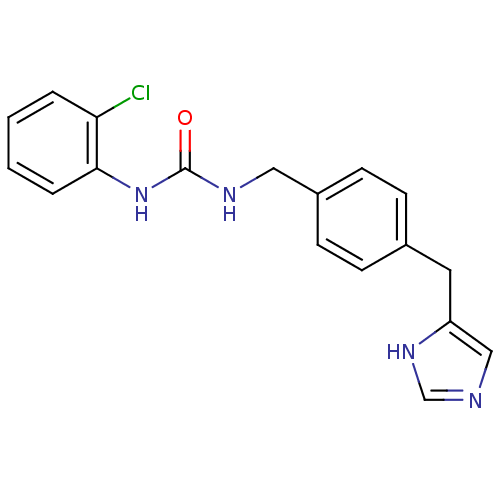 (1-(2-Chloro-phenyl)-3-[4-(1H-imidazol-4-ylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112027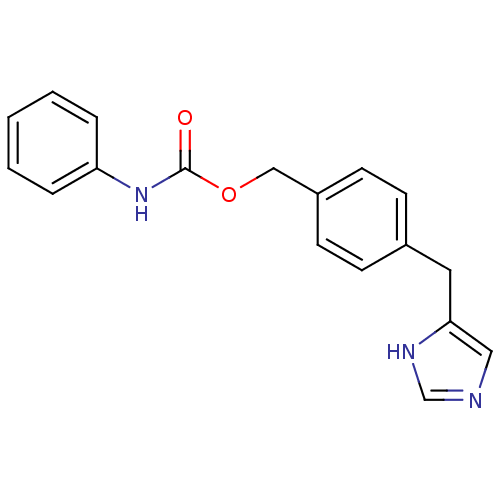 (CHEMBL172610 | Phenyl-carbamic acid 4-(1H-imidazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441513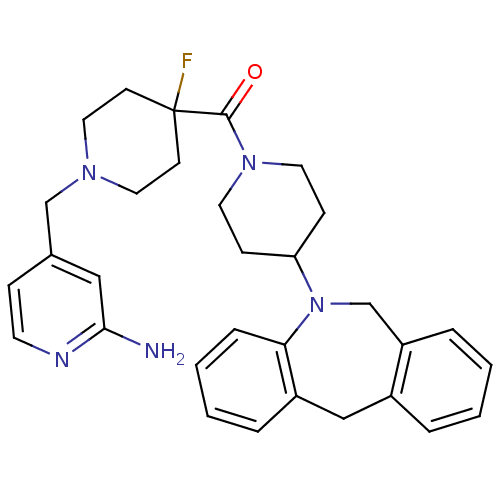 (CHEMBL2436616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441500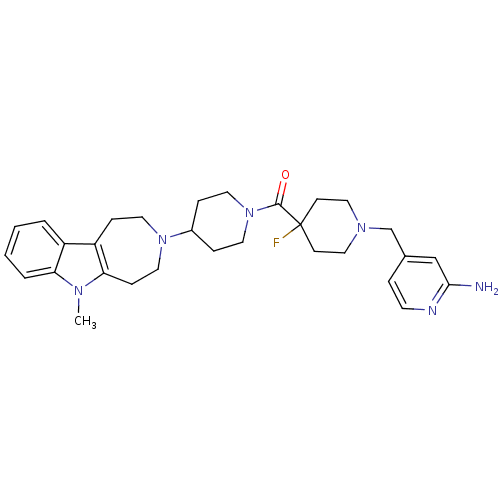 (CHEMBL2436632) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441516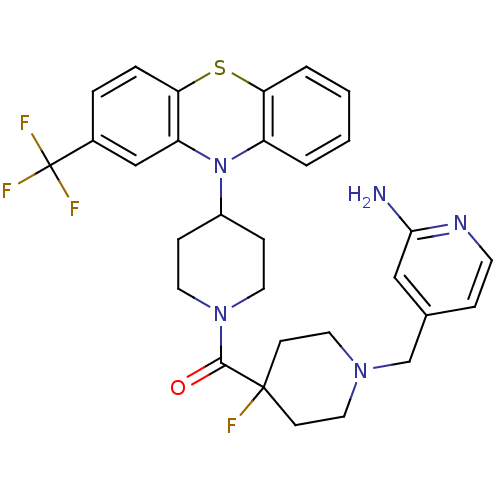 (CHEMBL2436618) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441510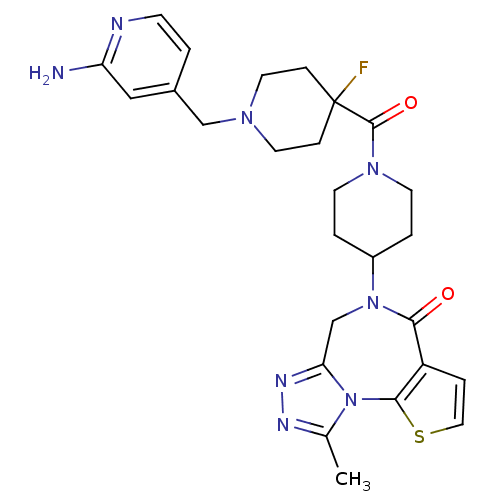 (CHEMBL2436621) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112026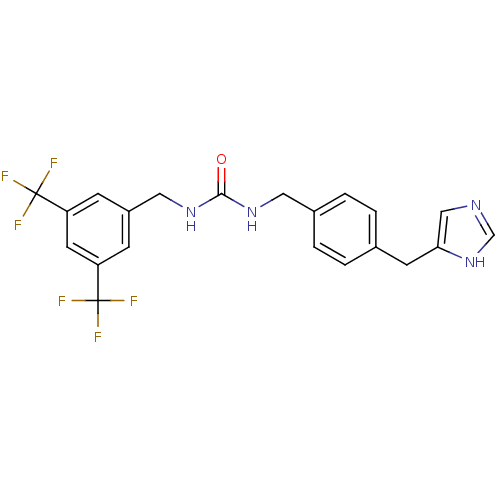 (1-(3,5-Bis-trifluoromethyl-benzyl)-3-[4-(1H-imidaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441508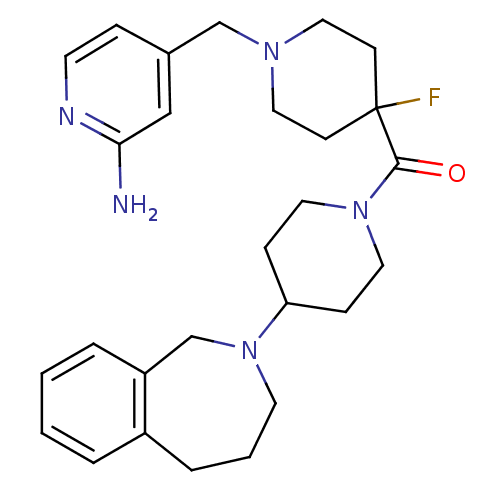 (CHEMBL2436623) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112023 (1-(3,5-Dichloro-phenyl)-3-[4-(1H-imidazol-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112022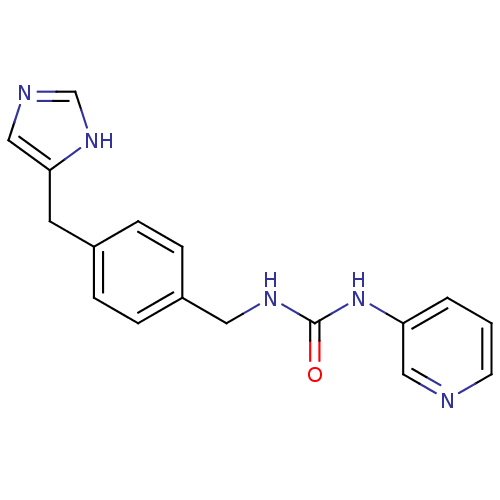 (1-[4-(1H-Imidazol-4-ylmethyl)-benzyl]-3-pyridin-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112014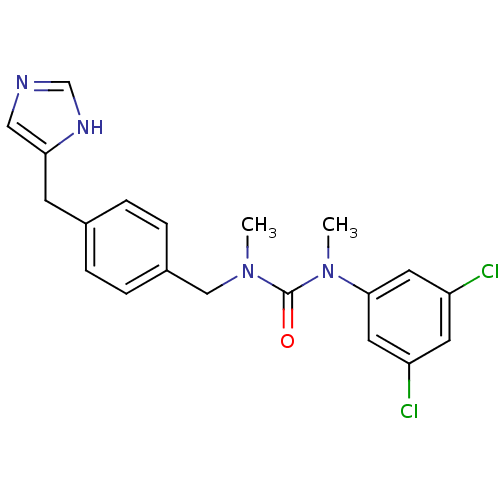 (1-(3,5-Dichloro-phenyl)-3-[4-(1H-imidazol-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50112015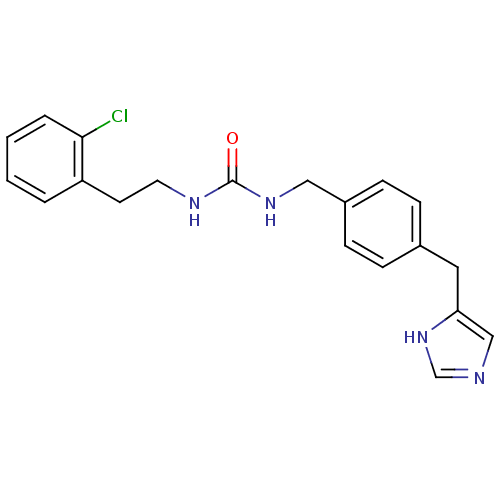 (1-[2-(2-Chloro-phenyl)-ethyl]-3-[4-(1H-imidazol-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Histamine H3 receptor of guinea pig brain membrane | Bioorg Med Chem Lett 12: 937-41 (2002) BindingDB Entry DOI: 10.7270/Q2W37VN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441514 (CHEMBL2436633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441513 (CHEMBL2436616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441513 (CHEMBL2436616) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441502 (CHEMBL2436629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441503 (CHEMBL2436628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441504 (CHEMBL2436627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441505 (CHEMBL2436626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441506 (CHEMBL2436625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441507 (CHEMBL2436624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441508 (CHEMBL2436623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |