 Found 77 hits with Last Name = 'bharath' and Initial = 's'
Found 77 hits with Last Name = 'bharath' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018442

(CHEMBL3286436)Show SMILES COc1cc(C#N)c2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2c1 Show InChI InChI=1S/C26H28N6O2/c1-18-20(14-27)11-19(16-29-18)17-30-22-5-7-31(8-6-22)9-10-32-25-13-23(34-2)12-21(15-28)24(25)3-4-26(32)33/h3-4,11-13,16,22,30H,5-10,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099957
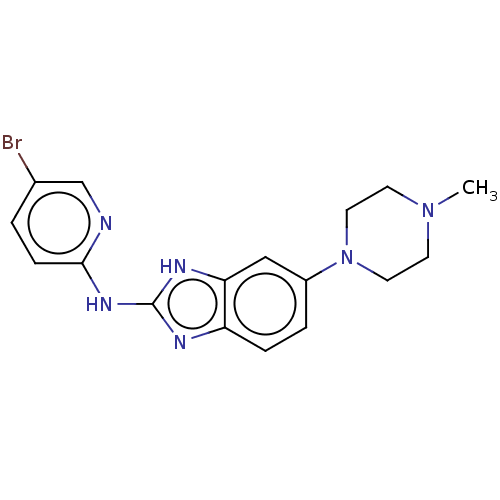
(CHEMBL3321871)Show InChI InChI=1S/C17H19BrN6/c1-23-6-8-24(9-7-23)13-3-4-14-15(10-13)21-17(20-14)22-16-5-2-12(18)11-19-16/h2-5,10-11H,6-9H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020413
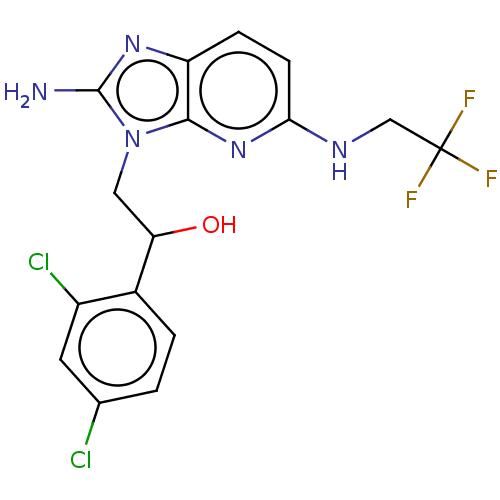
(CHEMBL3289807)Show SMILES Nc1nc2ccc(NCC(F)(F)F)nc2n1CC(O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H14Cl2F3N5O/c17-8-1-2-9(10(18)5-8)12(27)6-26-14-11(24-15(26)22)3-4-13(25-14)23-7-16(19,20)21/h1-5,12,27H,6-7H2,(H2,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100083
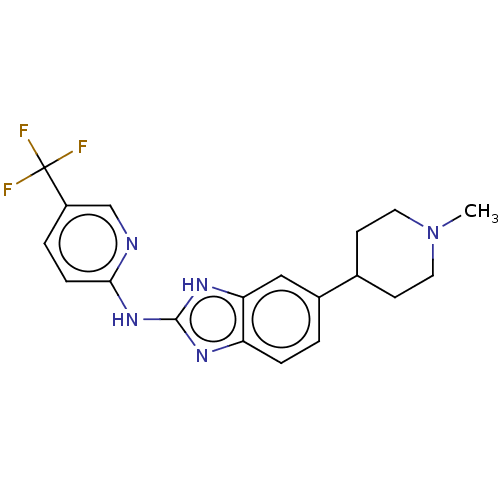
(CHEMBL3321972)Show SMILES CN1CCC(CC1)c1ccc2nc(Nc3ccc(cn3)C(F)(F)F)[nH]c2c1 Show InChI InChI=1S/C19H20F3N5/c1-27-8-6-12(7-9-27)13-2-4-15-16(10-13)25-18(24-15)26-17-5-3-14(11-23-17)19(20,21)22/h2-5,10-12H,6-9H2,1H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020414

(CHEMBL3289797)Show InChI InChI=1S/C14H15Cl2N3O/c15-9-3-4-10(11(16)5-9)13(20)7-19-6-12(8-1-2-8)18-14(19)17/h3-6,8,13,20H,1-2,7H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020415
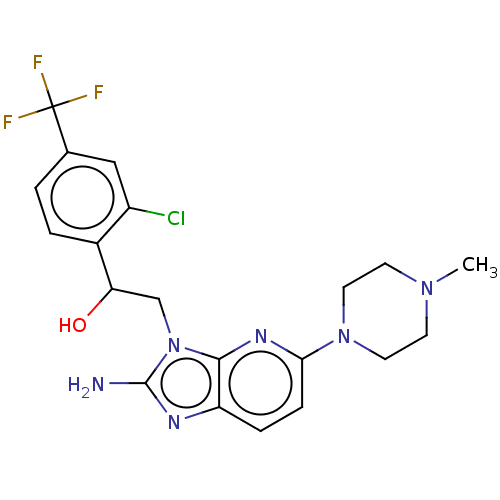
(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018290

(CHEMBL3290338)Show SMILES Cc1nc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)ccc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-21(16-27)4-6-23(29-18)17-28-22-8-10-30(11-9-22)12-13-31-24-14-19(15-26)2-3-20(24)5-7-25(31)32/h2-7,14,22,28H,8-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100015

(CHEMBL3321968)Show SMILES CN1CCN(CC1)c1cc(Cl)c2nc(Nc3ccc(Br)cn3)[nH]c2c1 Show InChI InChI=1S/C17H18BrClN6/c1-24-4-6-25(7-5-24)12-8-13(19)16-14(9-12)21-17(23-16)22-15-3-2-11(18)10-20-15/h2-3,8-10H,4-7H2,1H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100085

(CHEMBL3321980)Show SMILES CN1CCN(CC1)c1cc2[nH]c(Nc3cc(C4CC4)c(F)c(C)n3)nc2cn1 Show InChI InChI=1S/C20H24FN7/c1-12-19(21)14(13-3-4-13)9-17(23-12)26-20-24-15-10-18(22-11-16(15)25-20)28-7-5-27(2)6-8-28/h9-11,13H,3-8H2,1-2H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018440

(CHEMBL3290342)Show SMILES COc1ccc2n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c(=O)ccc2n1 Show InChI InChI=1S/C24H28N6O2/c1-17-19(14-25)13-18(15-26-17)16-27-20-7-9-29(10-8-20)11-12-30-22-4-5-23(32-2)28-21(22)3-6-24(30)31/h3-6,13,15,20,27H,7-12,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020416
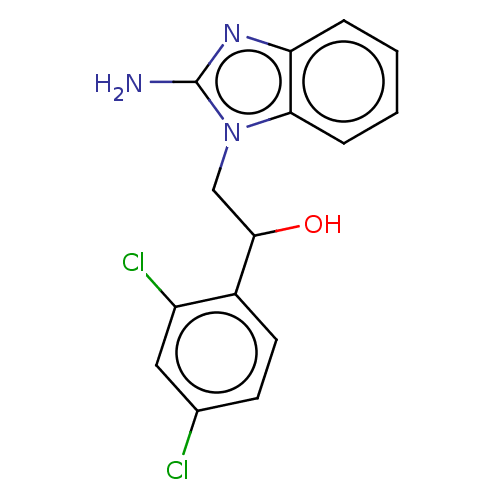
(CHEMBL3289799)Show InChI InChI=1S/C15H13Cl2N3O/c16-9-5-6-10(11(17)7-9)14(21)8-20-13-4-2-1-3-12(13)19-15(20)18/h1-7,14,21H,8H2,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018444
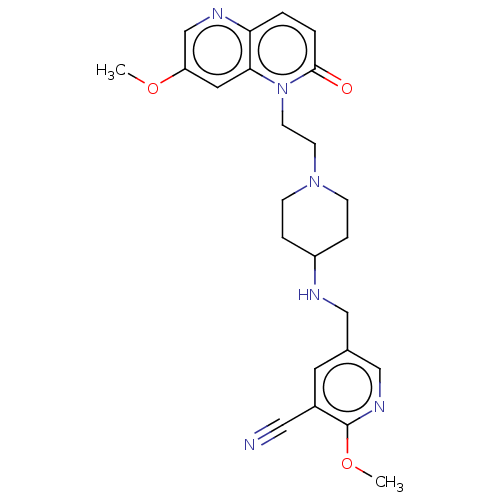
(CHEMBL3290345)Show SMILES COc1cnc2ccc(=O)n(CCN3CCC(CC3)NCc3cnc(OC)c(c3)C#N)c2c1 Show InChI InChI=1S/C24H28N6O3/c1-32-20-12-22-21(27-16-20)3-4-23(31)30(22)10-9-29-7-5-19(6-8-29)26-14-17-11-18(13-25)24(33-2)28-15-17/h3-4,11-12,15-16,19,26H,5-10,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099958

(CHEMBL3321873)Show InChI InChI=1S/C18H22N6/c1-13-3-6-17(19-12-13)22-18-20-15-5-4-14(11-16(15)21-18)24-9-7-23(2)8-10-24/h3-6,11-12H,7-10H2,1-2H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018447

(CHEMBL3290347)Show SMILES COc1ccc2ncc(=O)n(CCN3CCC(CC3)NCc3cnc(C)c(c3)C#N)c2n1 Show InChI InChI=1S/C23H27N7O2/c1-16-18(12-24)11-17(13-25-16)14-26-19-5-7-29(8-6-19)9-10-30-22(31)15-27-20-3-4-21(32-2)28-23(20)30/h3-4,11,13,15,19,26H,5-10,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018288
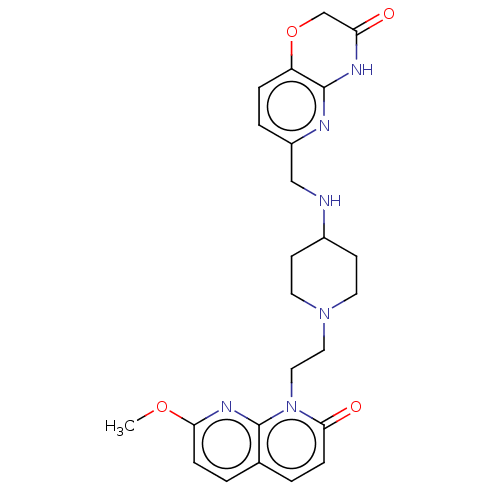
(CHEMBL3290336)Show SMILES COc1ccc2ccc(=O)n(CCN3CCC(CC3)NCc3ccc4OCC(=O)Nc4n3)c2n1 Show InChI InChI=1S/C24H28N6O4/c1-33-21-6-2-16-3-7-22(32)30(24(16)28-21)13-12-29-10-8-17(9-11-29)25-14-18-4-5-19-23(26-18)27-20(31)15-34-19/h2-7,17,25H,8-15H2,1H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100084
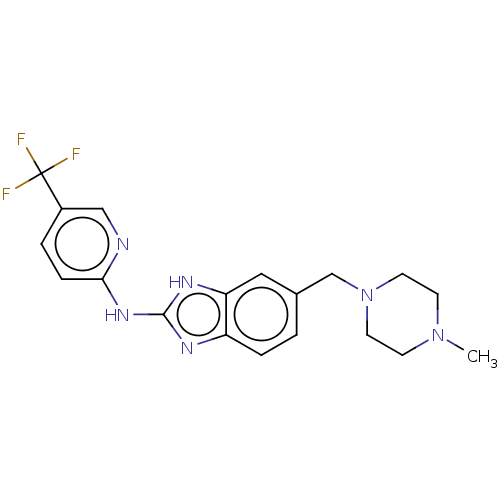
(CHEMBL3321963)Show SMILES CN1CCN(Cc2ccc3nc(Nc4ccc(cn4)C(F)(F)F)[nH]c3c2)CC1 Show InChI InChI=1S/C19H21F3N6/c1-27-6-8-28(9-7-27)12-13-2-4-15-16(10-13)25-18(24-15)26-17-5-3-14(11-23-17)19(20,21)22/h2-5,10-11H,6-9,12H2,1H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020417

(CHEMBL3289794)Show InChI InChI=1S/C11H11Cl2N3O/c12-8-3-1-2-7(10(8)13)9(17)6-16-5-4-15-11(16)14/h1-5,9,17H,6H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020418
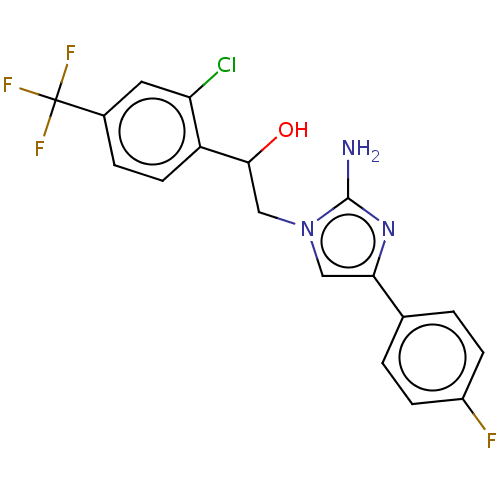
(CHEMBL3289798)Show SMILES Nc1nc(cn1CC(O)c1ccc(cc1Cl)C(F)(F)F)-c1ccc(F)cc1 Show InChI InChI=1S/C18H14ClF4N3O/c19-14-7-11(18(21,22)23)3-6-13(14)16(27)9-26-8-15(25-17(26)24)10-1-4-12(20)5-2-10/h1-8,16,27H,9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018407
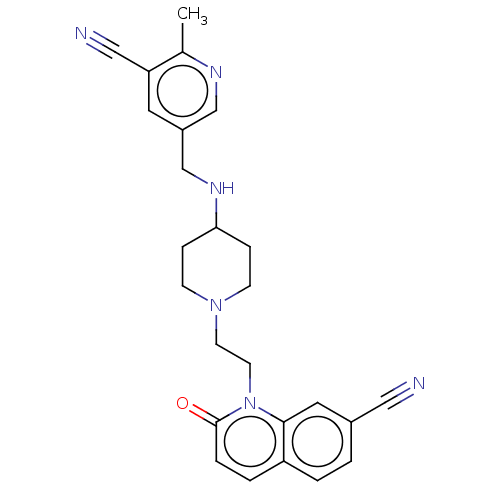
(CHEMBL3290339)Show SMILES Cc1ncc(CNC2CCN(CCn3c4cc(ccc4ccc3=O)C#N)CC2)cc1C#N Show InChI InChI=1S/C25H26N6O/c1-18-22(15-27)12-20(16-28-18)17-29-23-6-8-30(9-7-23)10-11-31-24-13-19(14-26)2-3-21(24)4-5-25(31)32/h2-5,12-13,16,23,29H,6-11,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50018441
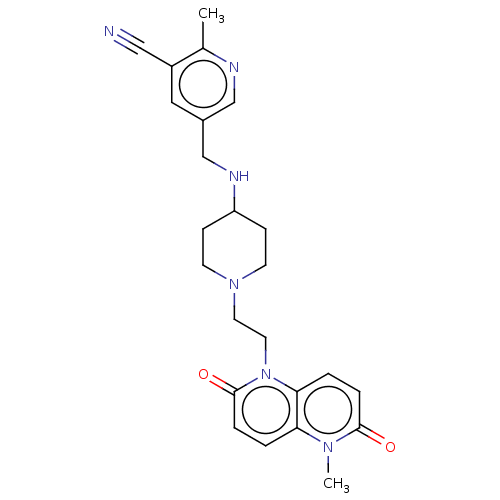
(CHEMBL3290343)Show SMILES Cc1ncc(CNC2CCN(CCn3c4ccc(=O)n(C)c4ccc3=O)CC2)cc1C#N Show InChI InChI=1S/C24H28N6O2/c1-17-19(14-25)13-18(15-26-17)16-27-20-7-9-29(10-8-20)11-12-30-22-4-5-23(31)28(2)21(22)3-6-24(30)32/h3-6,13,15,20,27H,7-12,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp electrophysiological analysis |
J Med Chem 57: 4889-905 (2014)
Article DOI: 10.1021/jm500432n
BindingDB Entry DOI: 10.7270/Q2416ZM4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020410
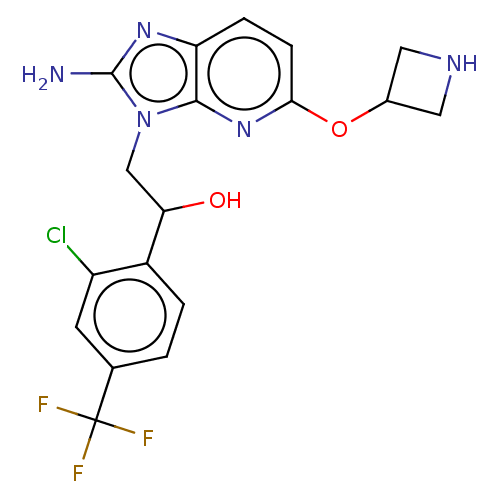
(CHEMBL3289812)Show SMILES Nc1nc2ccc(OC3CNC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C18H17ClF3N5O2/c19-12-5-9(18(20,21)22)1-2-11(12)14(28)8-27-16-13(25-17(27)23)3-4-15(26-16)29-10-6-24-7-10/h1-5,10,14,24,28H,6-8H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data