

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105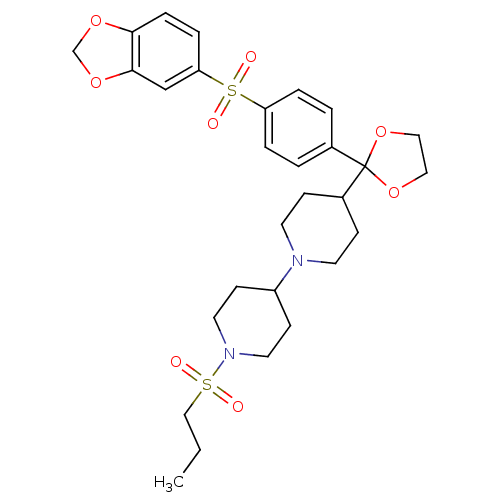 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. | Bioorg Med Chem Lett 10: 2727-30 (2000) BindingDB Entry DOI: 10.7270/Q2V40TGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069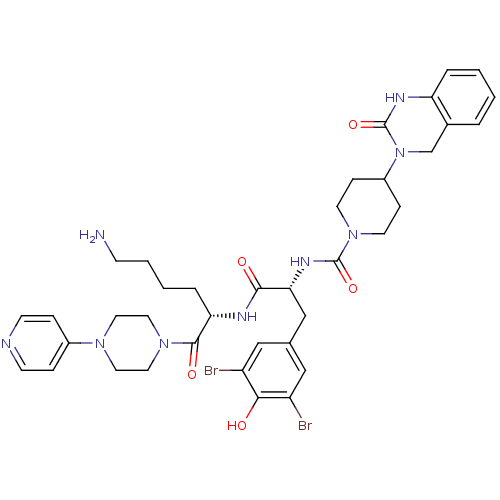 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476758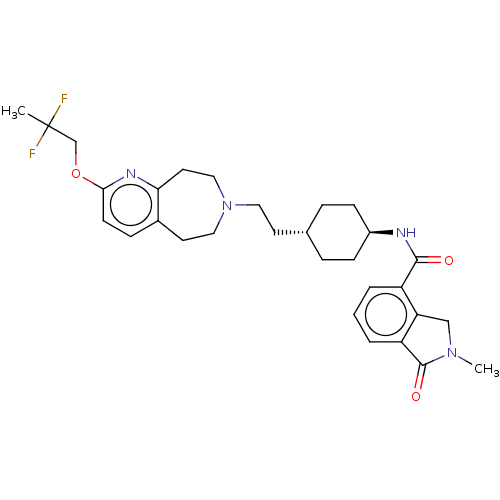 (US10870660, Compound III-024 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476918 (US10870660, Compound II-057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593894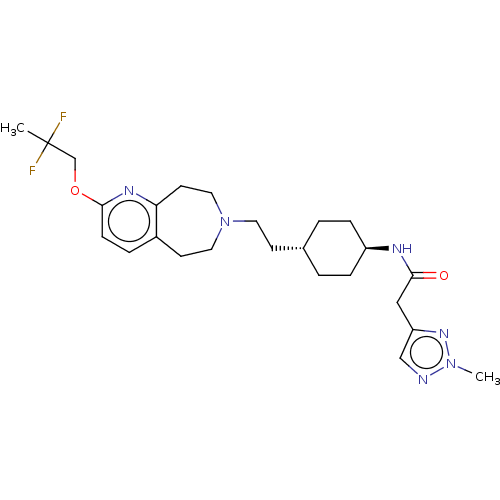 (US11578084, Compound I'-42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447327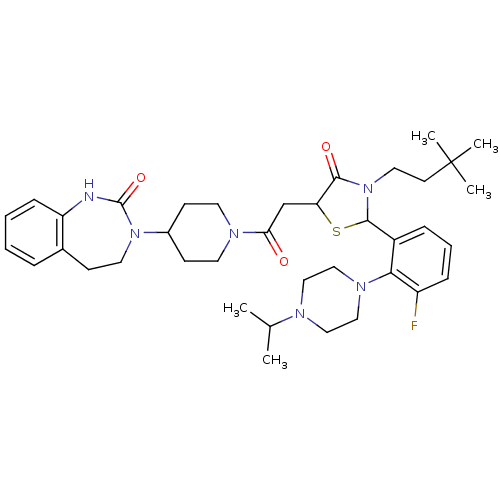 (CHEMBL3114495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476915 (US10870660, Compound II-047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593791 (US11578084, Compound I-074) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476764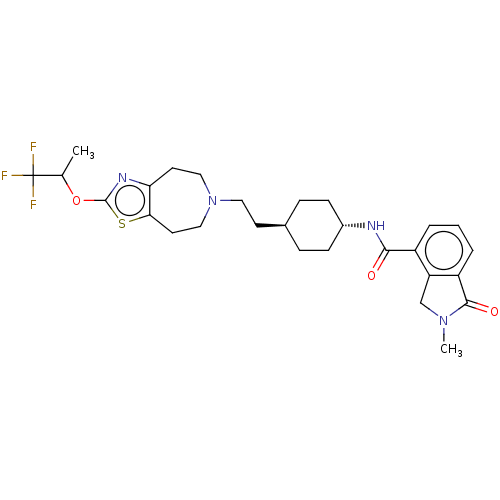 (US10870660, Compound III-064 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593940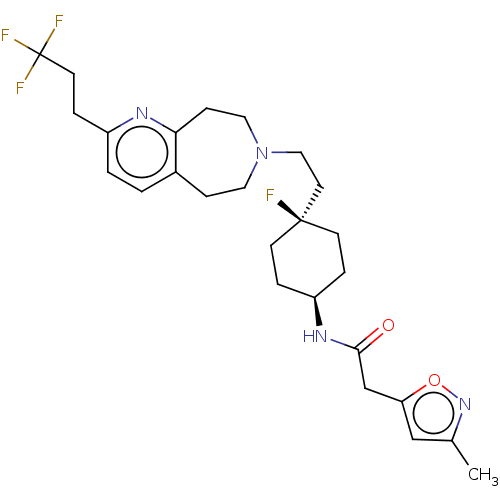 (US11578084, Compound III-3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593929 (US11578084, Compound I-157) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110523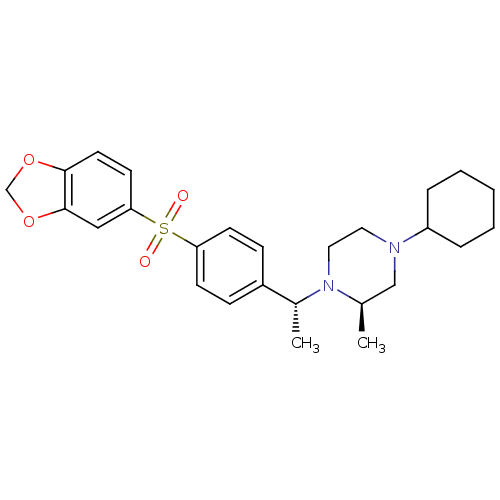 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110539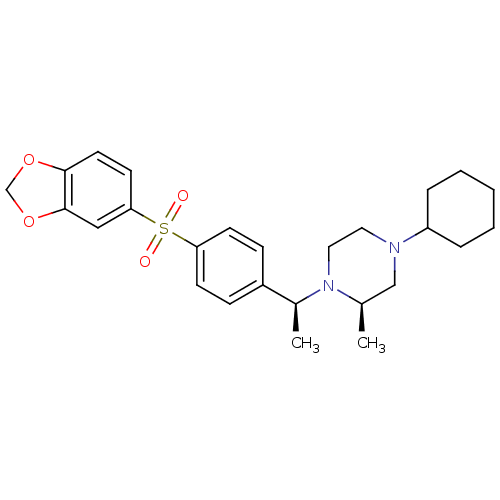 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447325 (CHEMBL3114676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451113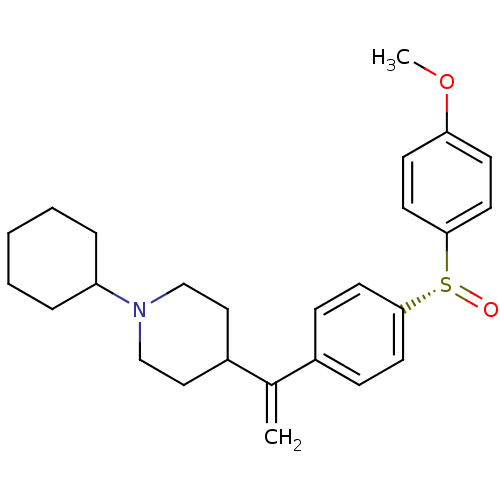 (CHEMBL2114068) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095111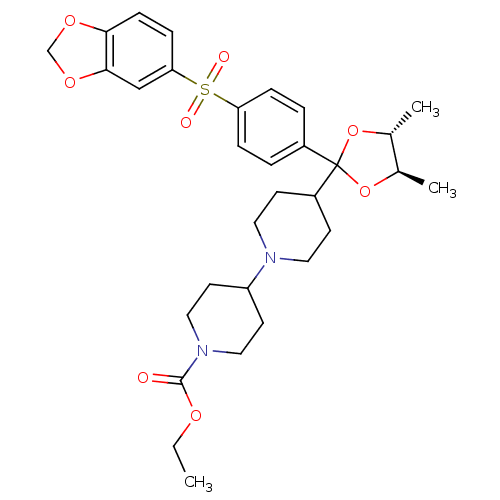 (4-{(4R,5R)-2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. | Bioorg Med Chem Lett 10: 2727-30 (2000) BindingDB Entry DOI: 10.7270/Q2V40TGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095097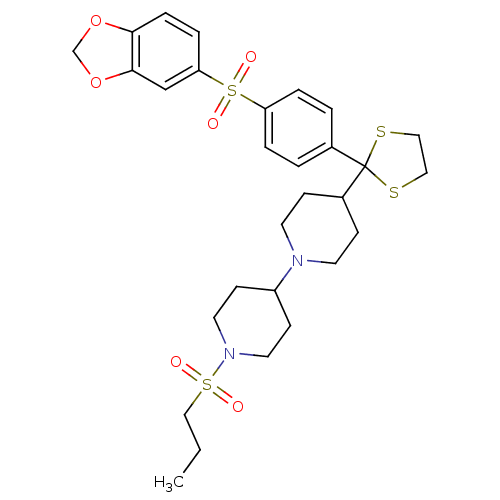 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. | Bioorg Med Chem Lett 10: 2727-30 (2000) BindingDB Entry DOI: 10.7270/Q2V40TGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593840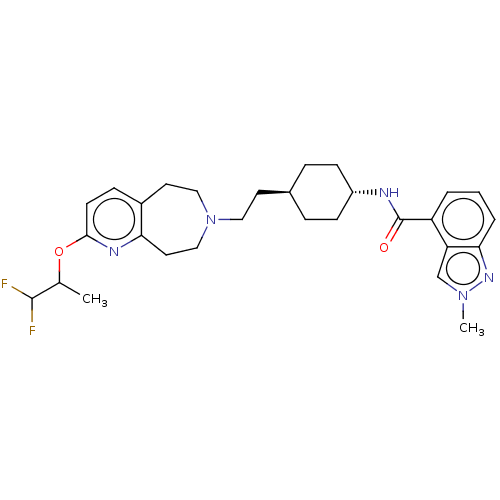 (US11578084, Compound I-123 | US11578084, Compound ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593735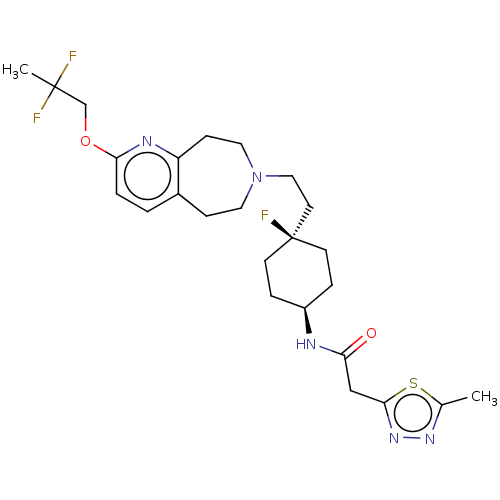 (US11578084, Compound I-018) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476836 (US10870660, Compound III-581 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593733 (US11578084, Compound I-016) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110534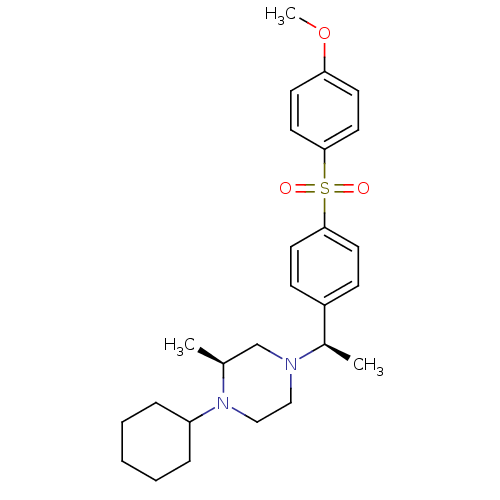 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593890 (US11578084, Compound I'-38) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593802 (US11578084, Compound I-085) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593765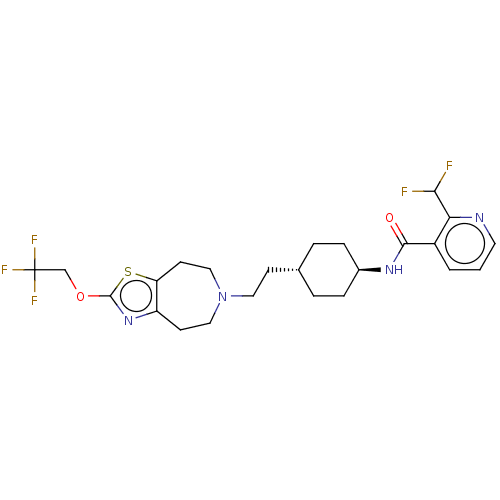 (US11578084, Compound I-048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476923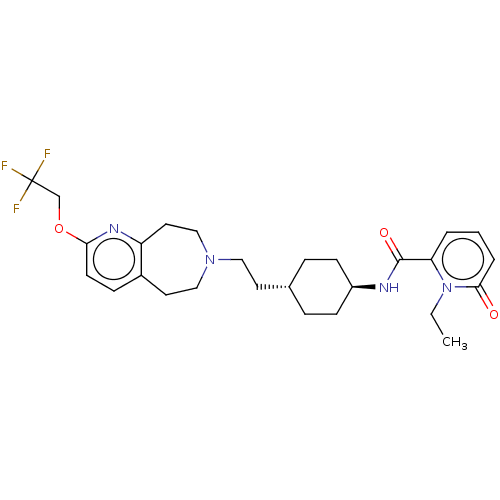 (US10870660, Compound II-081 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320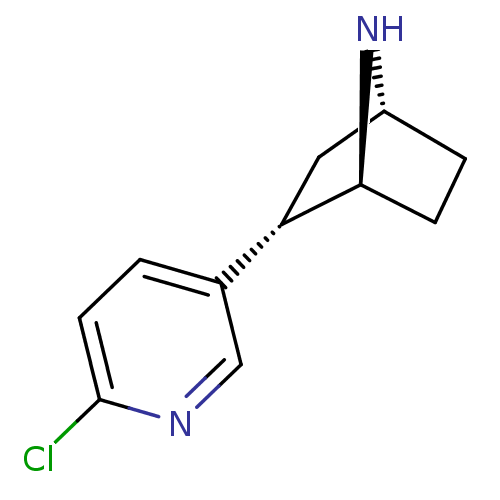 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology Zurich Curated by ChEMBL | Assay Description Displacement of [3H]-cytisine from Alpha4-beta2 Nicotinic acetylcholine receptor of rat brain homogenates | J Med Chem 48: 5123-30 (2005) Article DOI: 10.1021/jm040881a BindingDB Entry DOI: 10.7270/Q2PR7ZQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50171347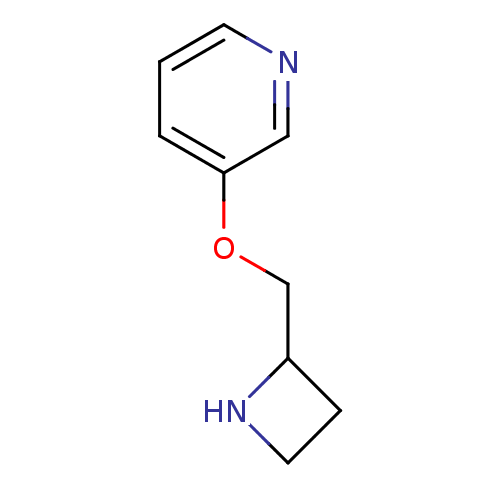 (3-(Azetidin-2-ylmethoxy)-pyridine | A-85380 | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology Zurich Curated by ChEMBL | Assay Description Displacement of [3H]-cytisine from Alpha4-beta2 Nicotinic acetylcholine receptor of rat brain homogenates | J Med Chem 48: 5123-30 (2005) Article DOI: 10.1021/jm040881a BindingDB Entry DOI: 10.7270/Q2PR7ZQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809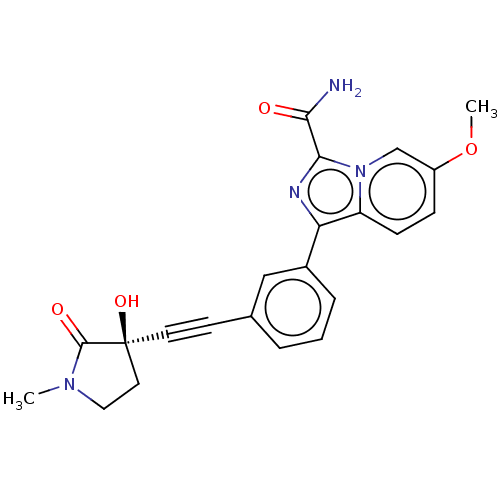 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476834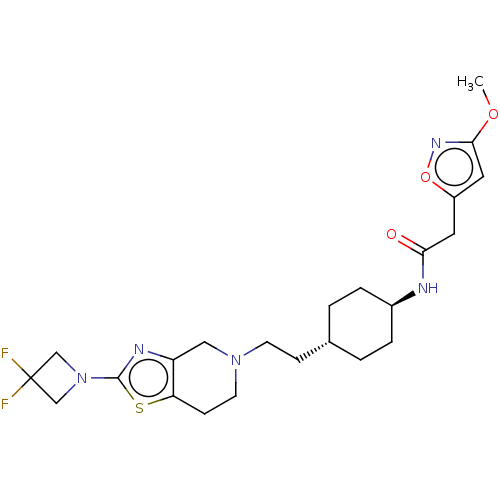 (US10870660, Compound III-578 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476858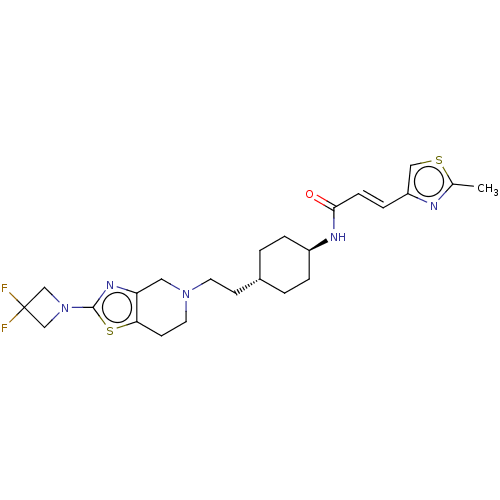 (US10870660, Compound III-706 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476924 (US10870660, Compound II-084 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476773 (US10870660, Compound III-138 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451114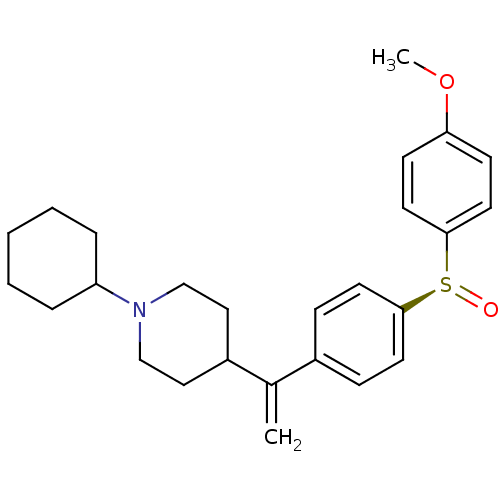 (CHEMBL2115128) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476914 (US10870660, Compound II-041) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593764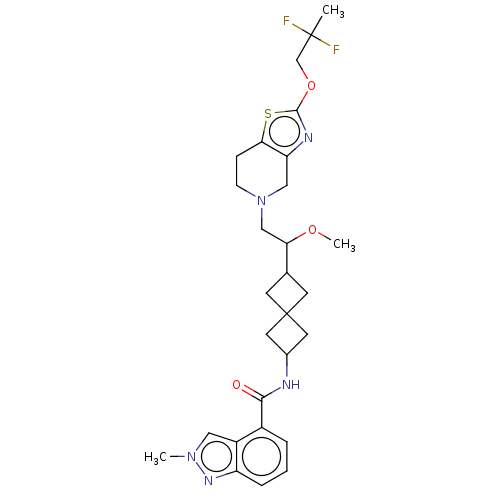 (US11578084, Compound I-047) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476925 (US10870660, Compound II-087) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332270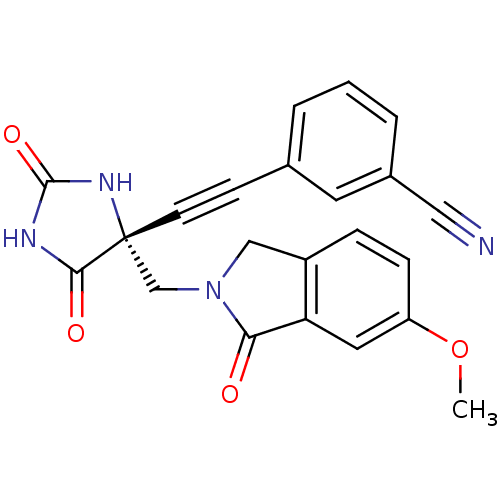 ((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of TACE | Bioorg Med Chem Lett 20: 7283-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.081 BindingDB Entry DOI: 10.7270/Q2Z89CP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593734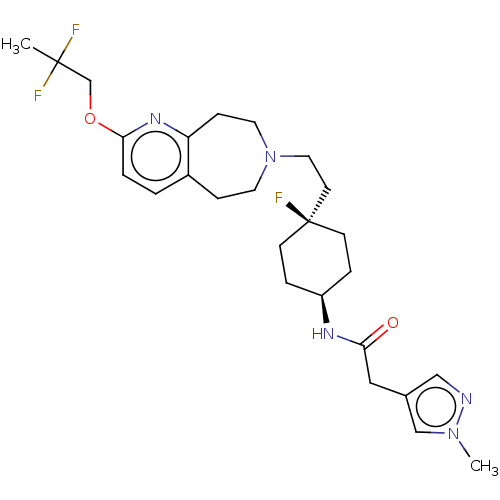 (US11578084, Compound I-017) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717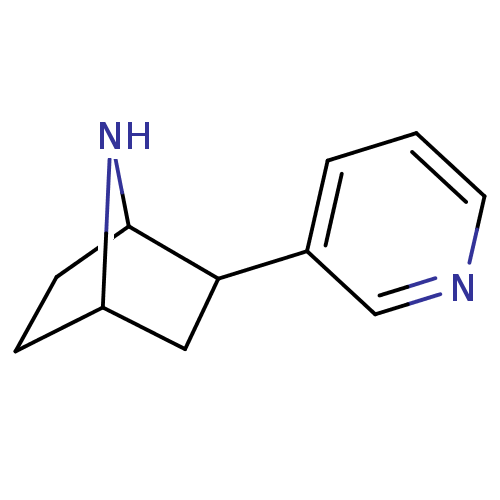 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology Zurich Curated by ChEMBL | Assay Description Displacement of [3H]-cytisine from Alpha4-beta2 Nicotinic acetylcholine receptor of rat brain homogenates | J Med Chem 48: 5123-30 (2005) Article DOI: 10.1021/jm040881a BindingDB Entry DOI: 10.7270/Q2PR7ZQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology Zurich Curated by ChEMBL | Assay Description Displacement of [3H]-cytisine from Alpha4-beta2 Nicotinic acetylcholine receptor of rat brain homogenates | J Med Chem 48: 5123-30 (2005) Article DOI: 10.1021/jm040881a BindingDB Entry DOI: 10.7270/Q2PR7ZQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476759 (US10870660, Compound III-027 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50121132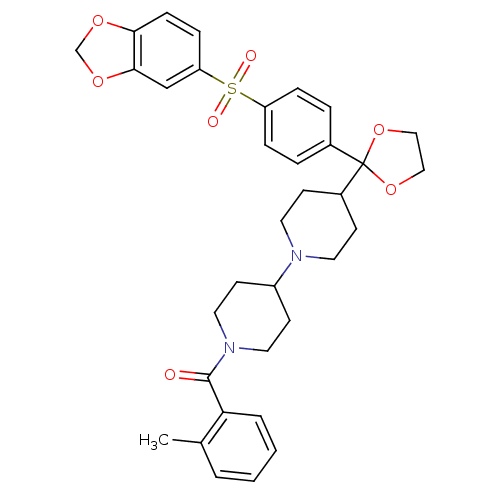 ((4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 12: 3479-82 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476866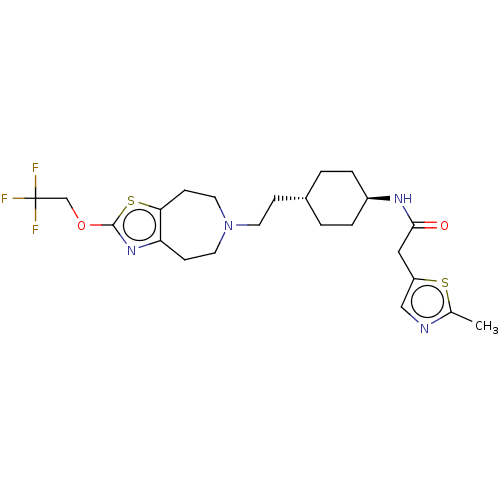 (US10870660, Compound II-053 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50092313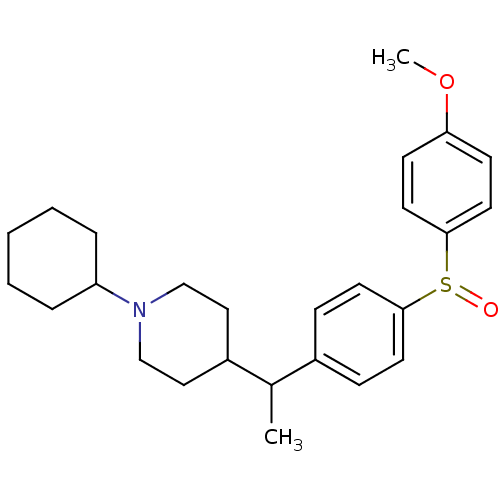 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M4 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092959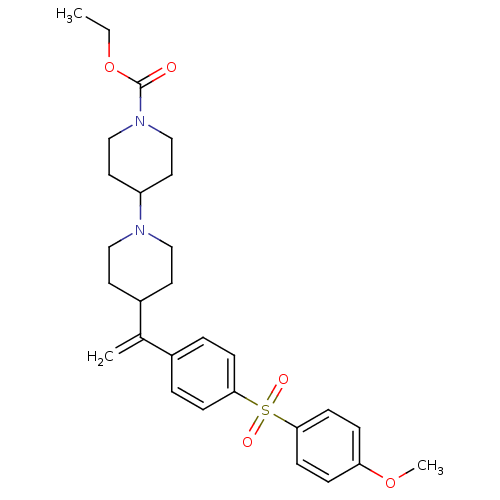 (4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. | Bioorg Med Chem Lett 10: 2727-30 (2000) BindingDB Entry DOI: 10.7270/Q2V40TGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457816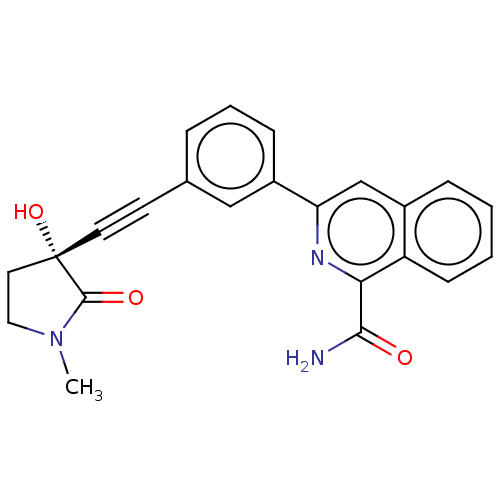 (CHEMBL4215425) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11608 total ) | Next | Last >> |