

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50071565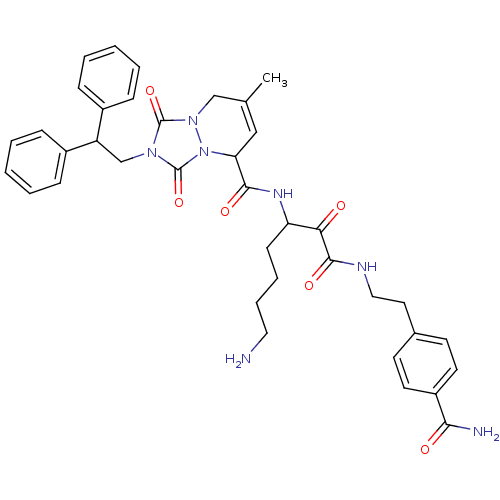 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071575 (2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071573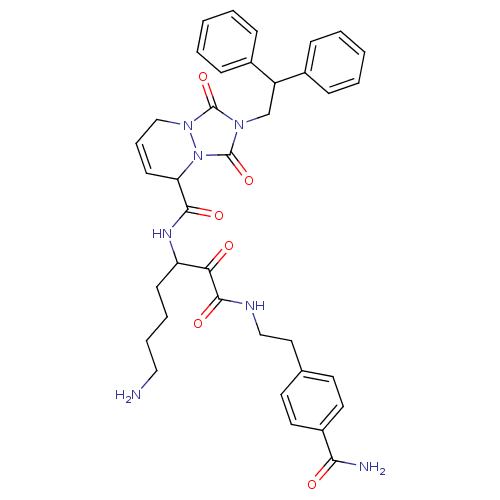 (2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571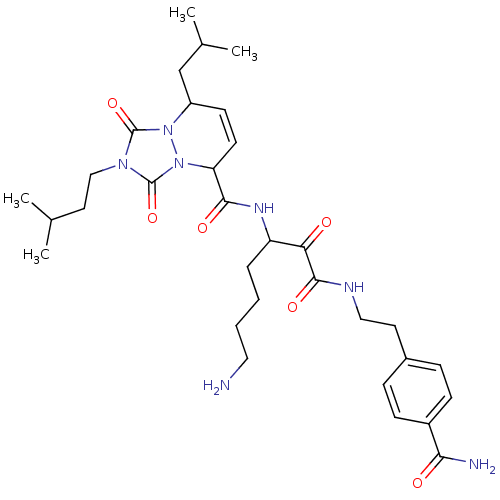 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071568 (2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071567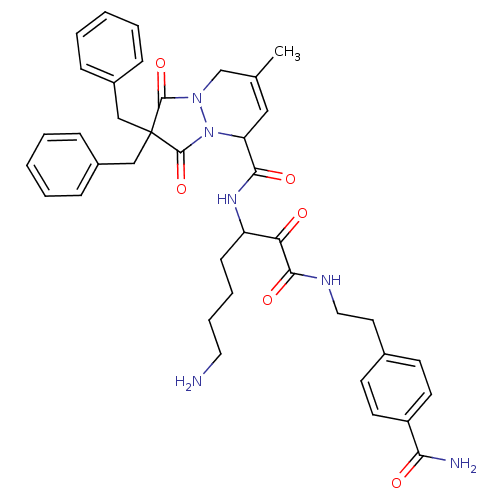 (2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071566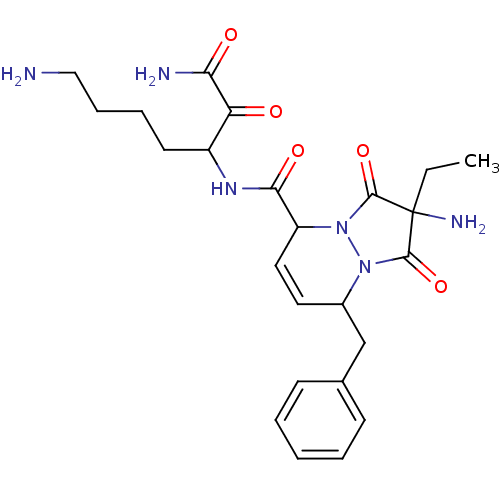 (2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071574 (2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50216213 (CHEMBL306744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the tryptase | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071564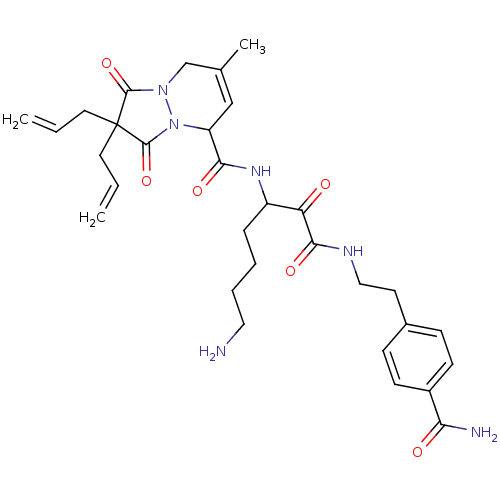 (2,2-Diallyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50071572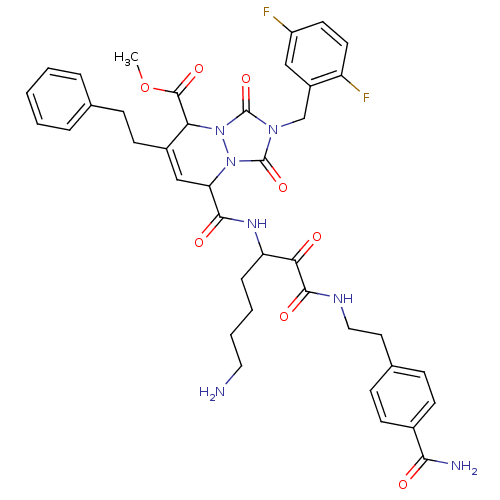 (8-{5-Amino-1-[2-(4-carbamoyl-phenyl)-ethylaminooxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Kallikrein | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50071563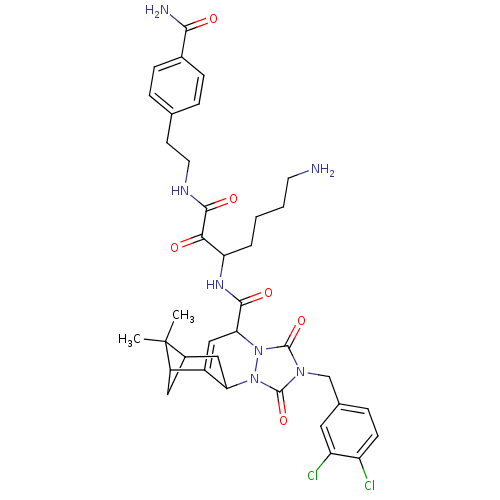 (4-(2-{6-amino-2-[7-(3,4-dichlorobenzyl)-13,13-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Kallikrein | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50511058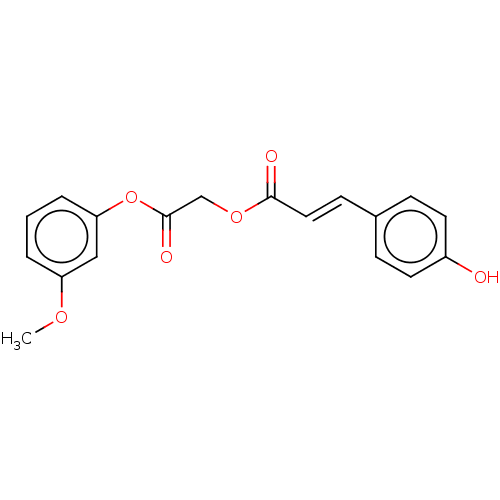 (CHEMBL4453514) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland (UQ) Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition and further incub... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126722 BindingDB Entry DOI: 10.7270/Q29S1VBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50511056 (CHEMBL4578912) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland (UQ) Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition and further incub... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126722 BindingDB Entry DOI: 10.7270/Q29S1VBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50608336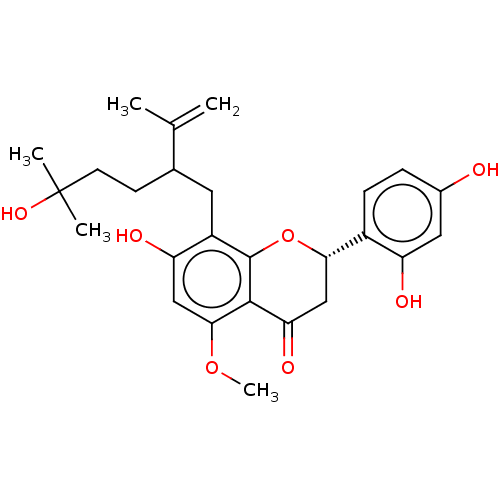 (CHEMBL517832) | UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13307 (1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-{2-[(...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Duke University Medical Center | Assay Description Assays for PfPFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). An amount of 1 uM ... | Chem Biol 16: 181-92 (2009) Article DOI: 10.1016/j.chembiol.2009.01.014 BindingDB Entry DOI: 10.7270/Q22F7KR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells | J Med Chem 45: 177-88 (2001) BindingDB Entry DOI: 10.7270/Q22F7MQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 0.613 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50045000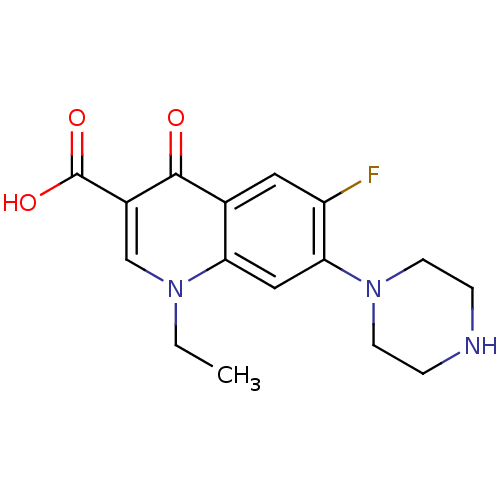 ((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50241367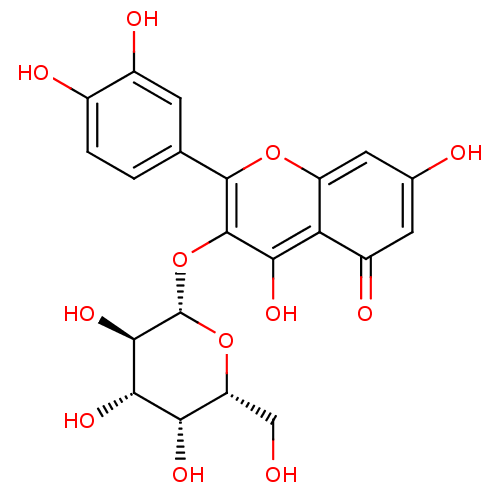 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-((2S,4R,5...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 0.762 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50217942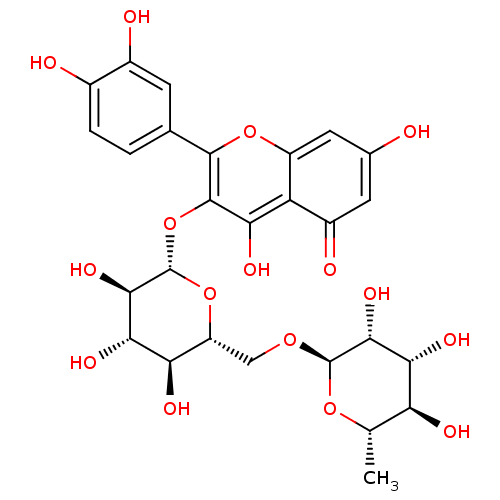 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 0.856 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50378581 (NICOTIFLOROSIDE) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | n/a | n/a | 0.908 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50208612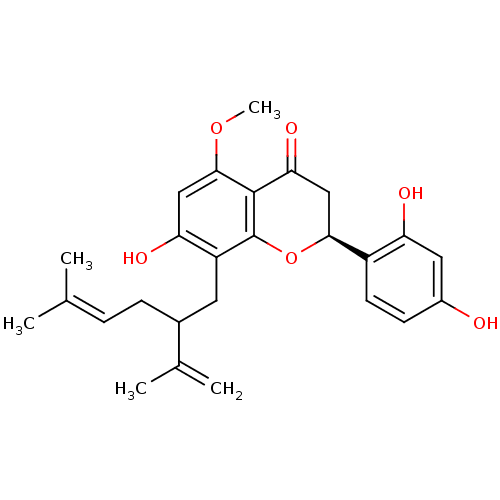 ((-)-kurarinone | (2S)-2-(2,4-dihydroxyphenyl)-7-hy...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM16182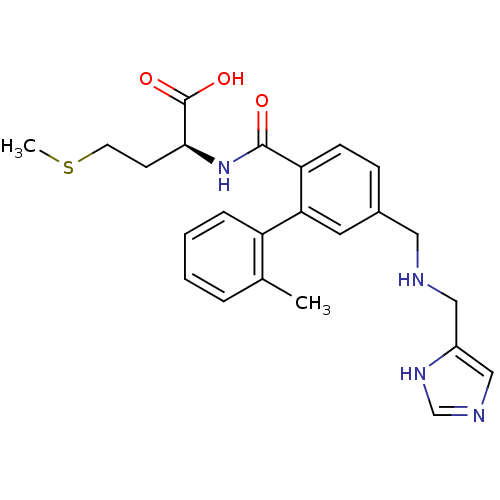 ((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM31423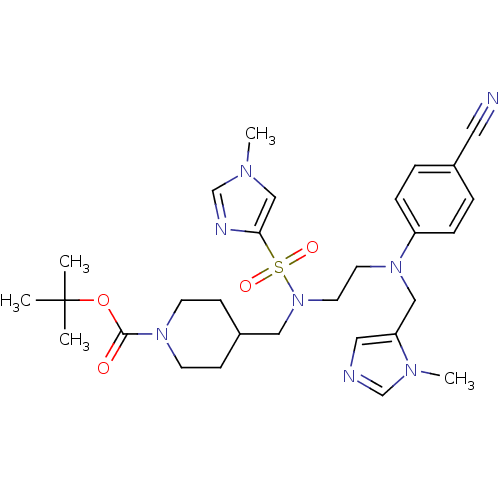 (ethylenediamine scaffold, 4) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Duke University Medical Center | Assay Description Assays for PfPFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). An amount of 1 uM ... | Chem Biol 16: 181-92 (2009) Article DOI: 10.1016/j.chembiol.2009.01.014 BindingDB Entry DOI: 10.7270/Q22F7KR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50366824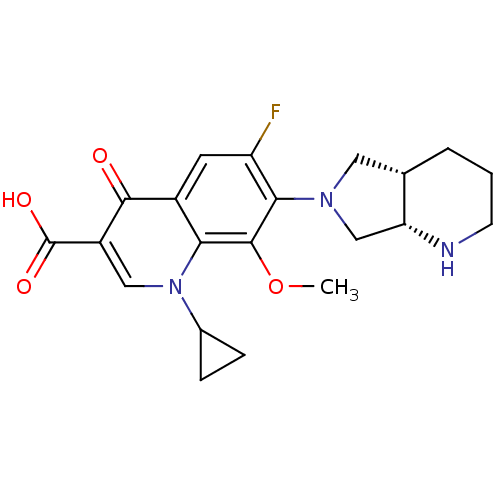 (Avelox | MOXIFLOXACIN | Moxifl-oxacin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50608335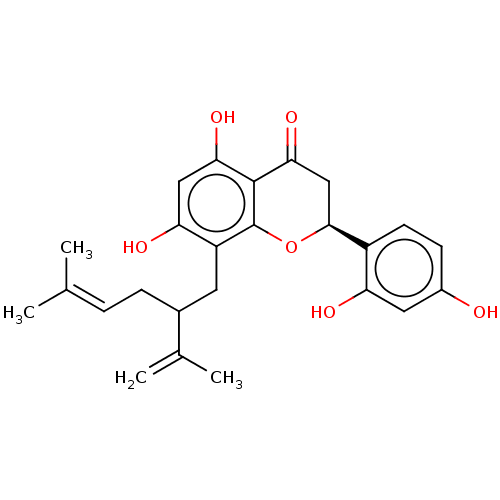 (CHEMBL479477) | UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM31424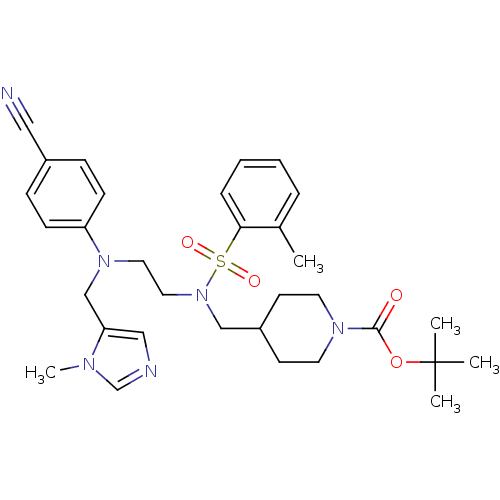 (ethylenediamine scaffold, 5) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Duke University Medical Center | Assay Description Assays for PfPFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). An amount of 1 uM ... | Chem Biol 16: 181-92 (2009) Article DOI: 10.1016/j.chembiol.2009.01.014 BindingDB Entry DOI: 10.7270/Q22F7KR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM31425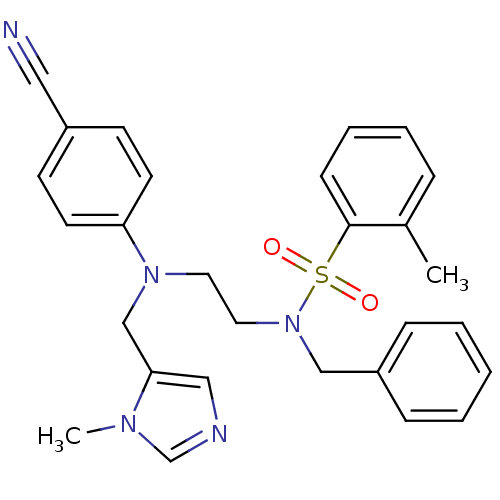 (ethylenediamine scaffold, 7) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Duke University Medical Center | Assay Description Assays for PfPFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). An amount of 1 uM ... | Chem Biol 16: 181-92 (2009) Article DOI: 10.1016/j.chembiol.2009.01.014 BindingDB Entry DOI: 10.7270/Q22F7KR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097809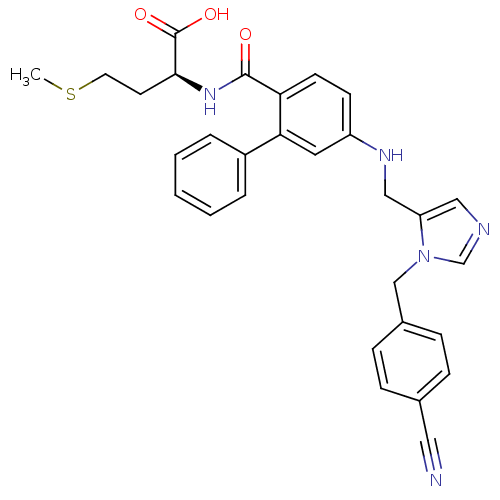 ((S)-2-[(5-{[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50076142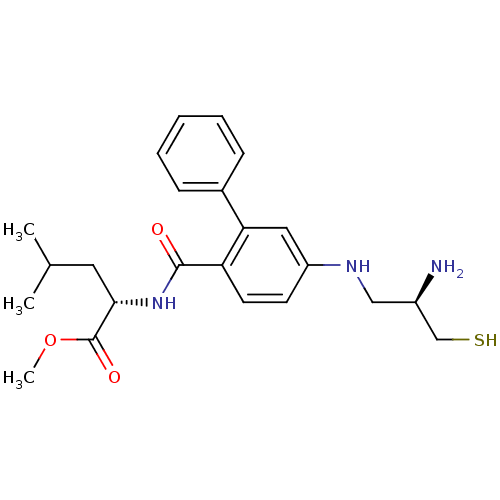 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-GGPP incorporation into H-Ras-CVLL by Geranylgeranyl transferase type I | J Med Chem 42: 1333-40 (1999) Article DOI: 10.1021/jm9900873 BindingDB Entry DOI: 10.7270/Q28G8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM230658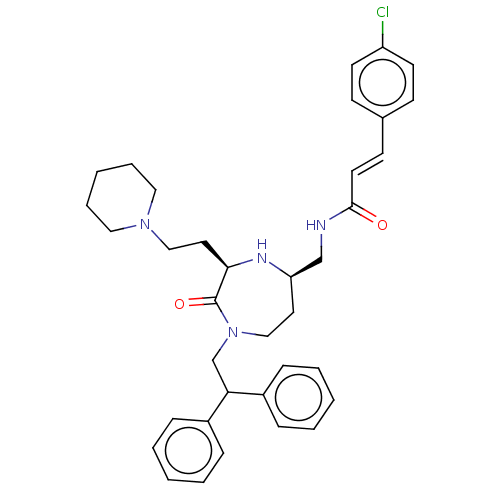 (US9340517, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM231897 (US9340517, 284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM231900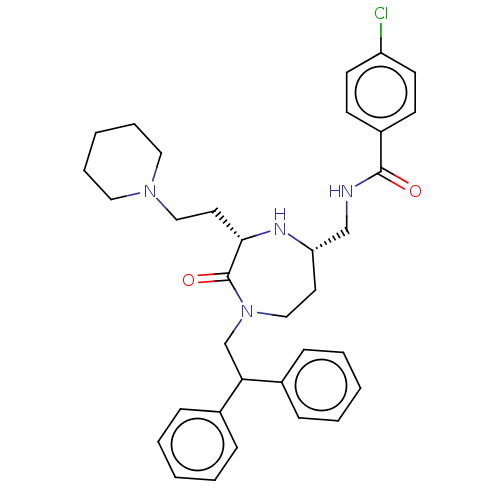 (US9340517, 287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM231794 (US9340517, 181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM231823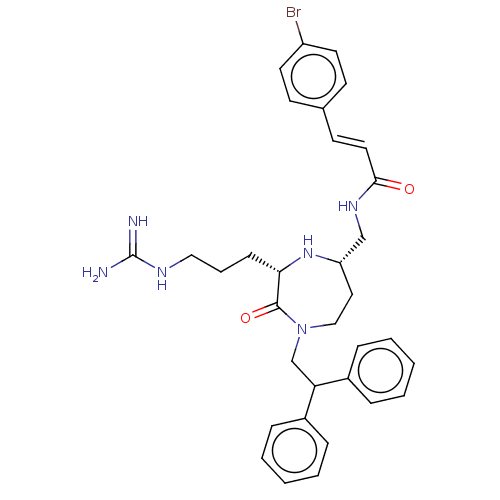 (US9340517, 210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM230658 (US9340517, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM231712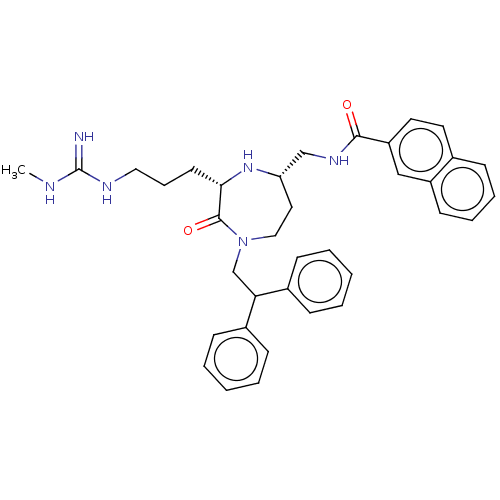 (US9340517, 67) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232162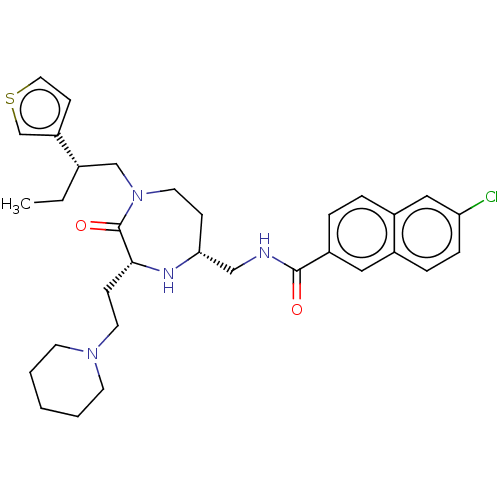 (US9340517, 550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232177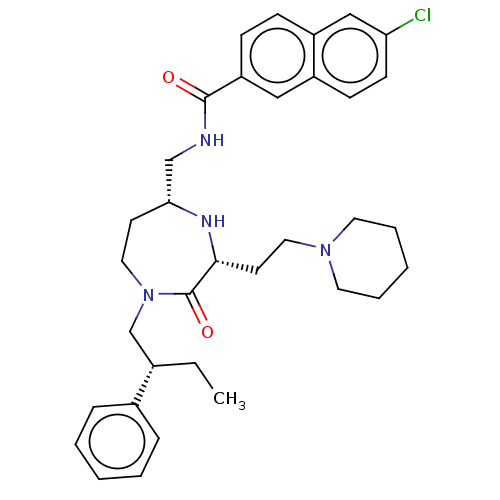 (US9340517, 565 | US9340517, 580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232179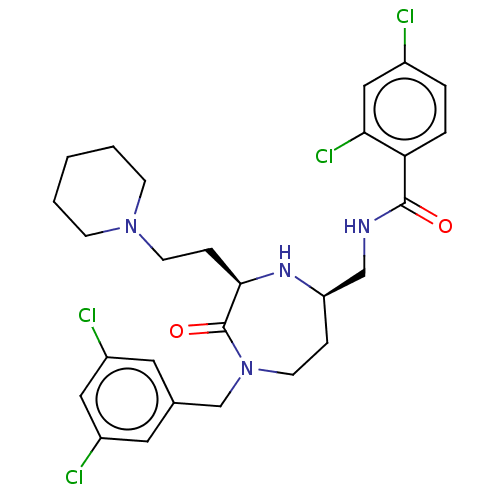 (US9340517, 567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232037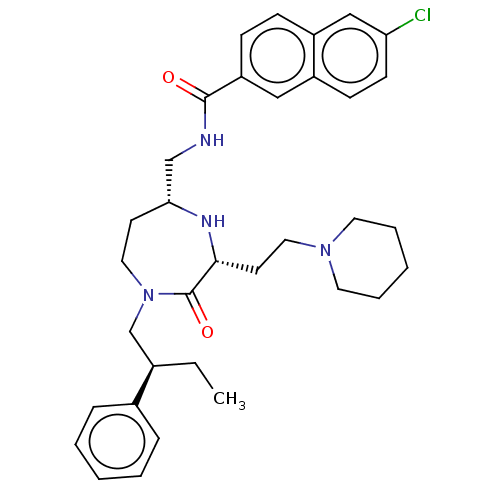 (US9340517, 425 | US9340517, 569 | US9340517, 579 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232183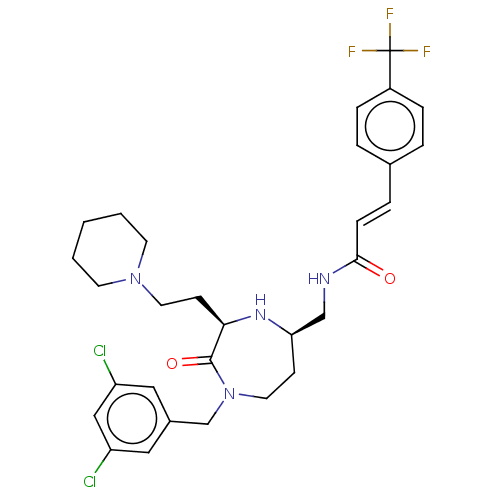 (US9340517, 571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232184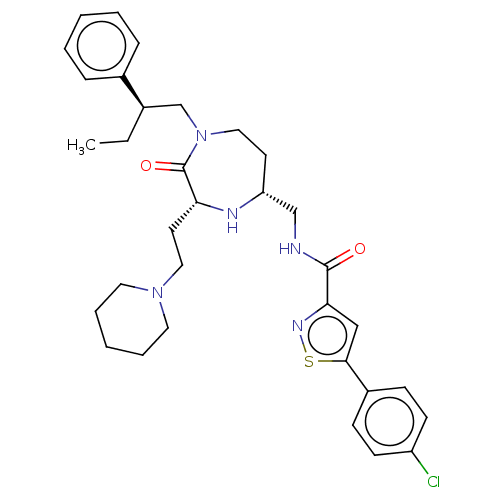 (US9340517, 572) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232185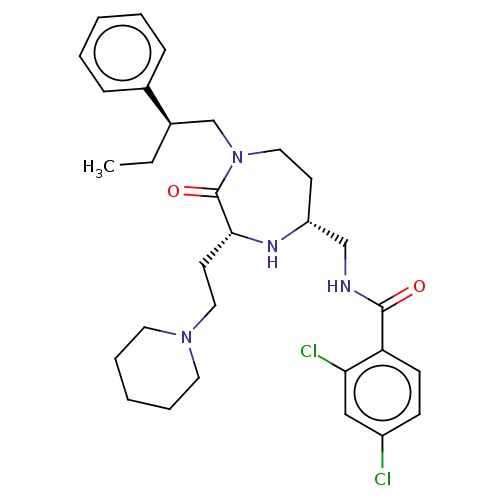 (US9340517, 573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232190 (US9340517, 578) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM232037 (US9340517, 425 | US9340517, 569 | US9340517, 579 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Mimetica PTY LTD US Patent | Assay Description Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially ... | US Patent US9340517 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 906 total ) | Next | Last >> |