

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50020284 (CHEMBL3288833) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020294 (CHEMBL3288834) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020278 (CHEMBL3288827) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020283 (CHEMBL3288832) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020307 (CHEMBL3288835) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020276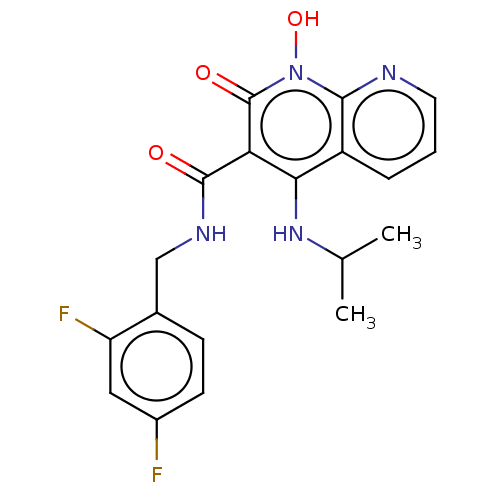 (CHEMBL3288825) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020281 (CHEMBL3288830) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020259 (CHEMBL3288817) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020309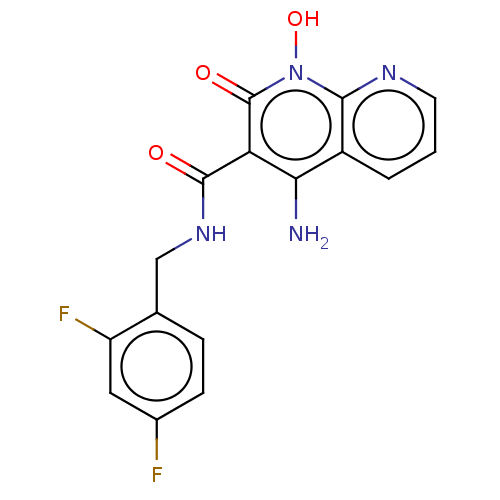 (CHEMBL3288837) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020280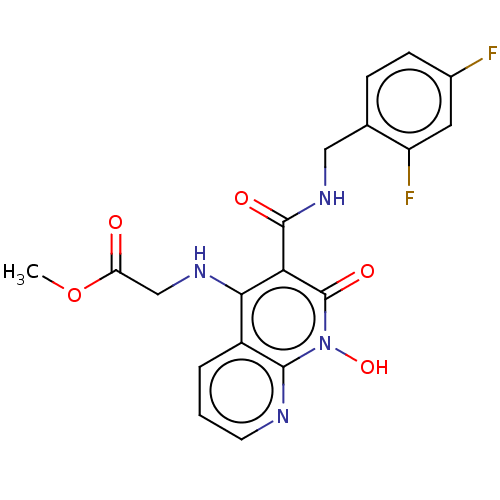 (CHEMBL3288829) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020267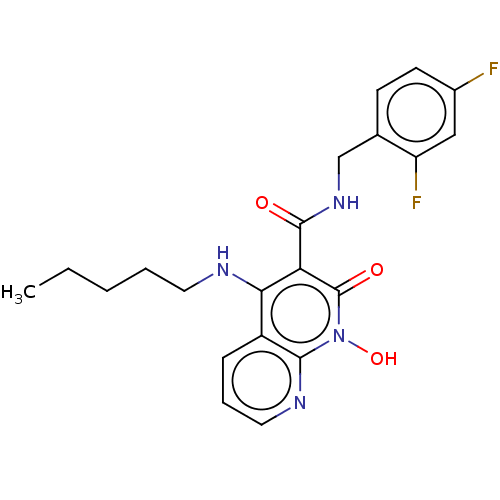 (CHEMBL3288824) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020282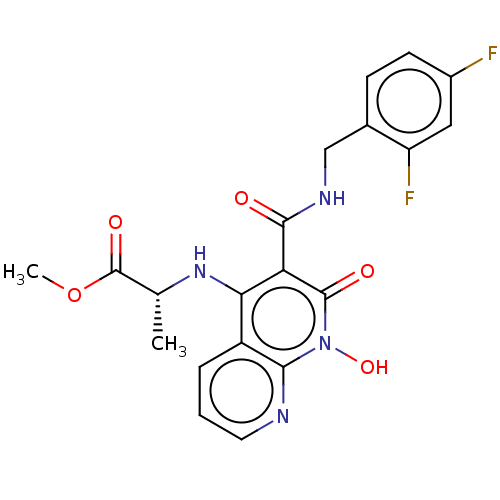 (CHEMBL3288831) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020279 (CHEMBL3288828) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020260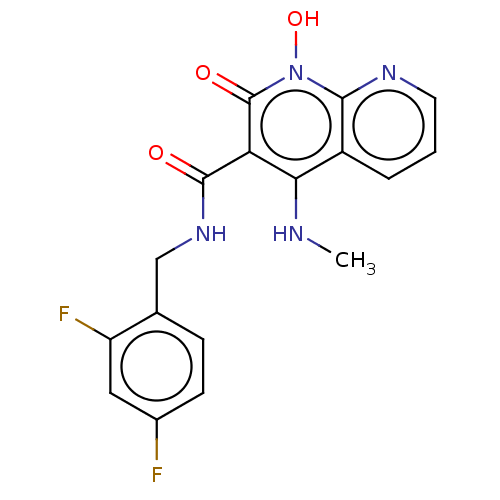 (CHEMBL3288818) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020277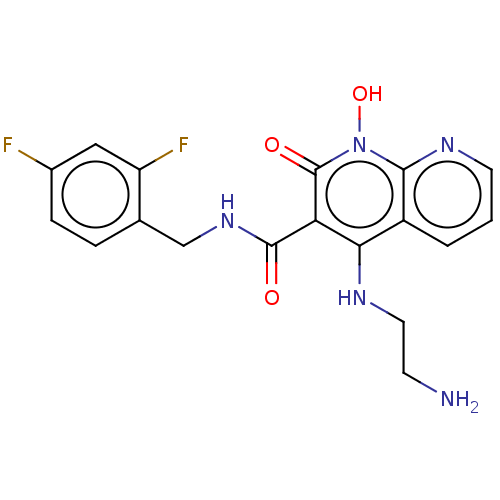 (CHEMBL3288826) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020265 (CHEMBL3288822) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020263 (CHEMBL3288820) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50130699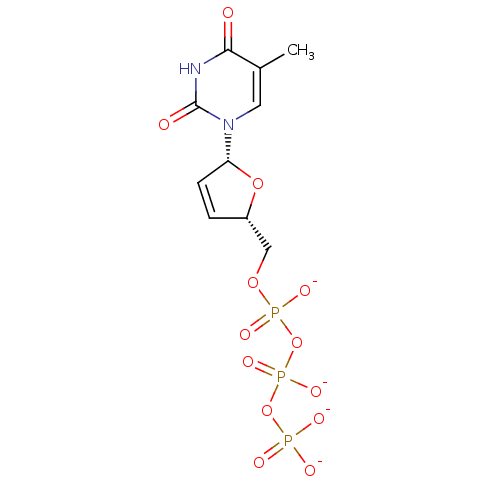 (1-(5-Hydroxymethyl-2,5-dihydro-furan-2-yl)-5-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibitory concentration required against Reverse transcriptase (RT) | J Med Chem 46: 3292-9 (2003) Article DOI: 10.1021/jm030116g BindingDB Entry DOI: 10.7270/Q2PR7WRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020262 (CHEMBL3288819) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481090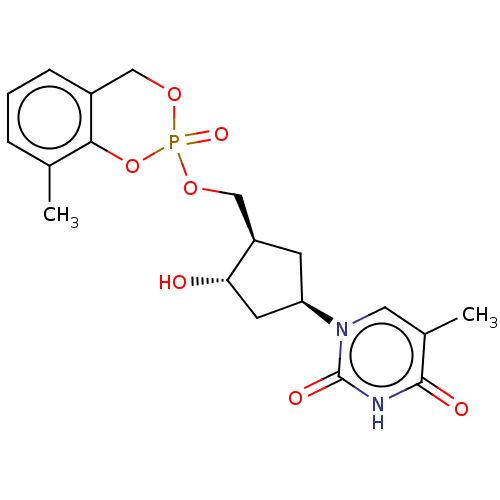 (CHEMBL567882) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase-mediated viral DNA synthesis in human HOS-313 cells expressing HSV thymidine kinase assessed as lu... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020257 (CHEMBL3288816) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020256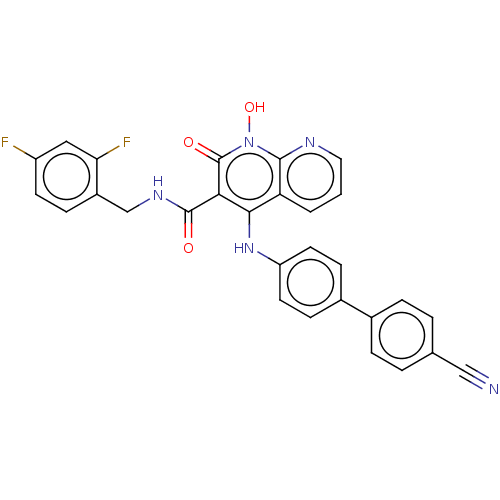 (CHEMBL3288815) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50481091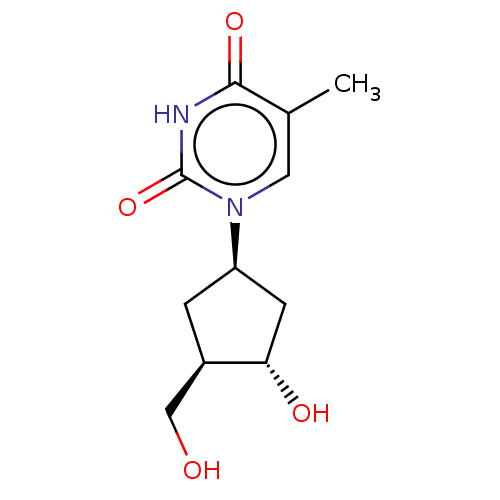 (CHEMBL27473) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M184V mutant-mediated viral DNA synthesis in human HOS-313 cells expressing HSV thymidine kinase assessed as... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020266 (CHEMBL3288823) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020308 (CHEMBL3288836) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020252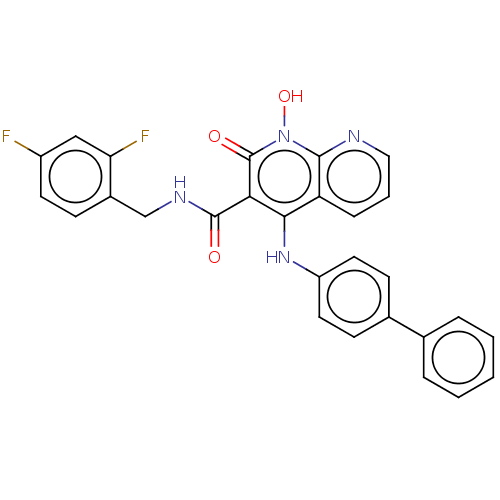 (CHEMBL3288813) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481090 (CHEMBL567882) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase-mediated viral DNA synthesis in human HOS cells assessed as luciferase activity treated 3 hrs befo... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020264 (CHEMBL3288821) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM10245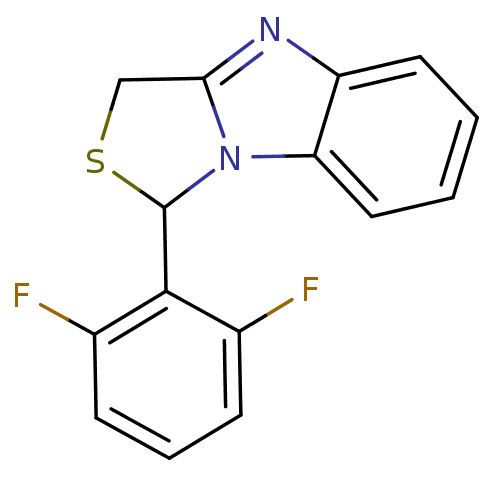 (3-(2,6-difluorophenyl)-4-thia-2,7-diazatricyclo[6....) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description The quantity of compound required to reduce WT Reverse transcriptase activity by 50% | J Med Chem 40: 4199-207 (1998) Article DOI: 10.1021/jm970096g BindingDB Entry DOI: 10.7270/Q25B01KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020278 (CHEMBL3288827) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50130700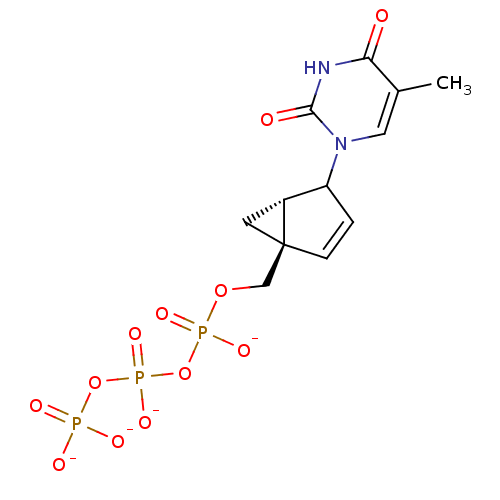 (1-(5-Hydroxymethyl-bicyclo[3.1.0]hex-3-en-2-yl)-5-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibitory concentration required against Reverse transcriptase (RT) | J Med Chem 46: 3292-9 (2003) Article DOI: 10.1021/jm030116g BindingDB Entry DOI: 10.7270/Q2PR7WRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020280 (CHEMBL3288829) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481091 (CHEMBL27473) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase-mediated viral DNA synthesis in human HOS-313 cells expressing HSV thymidine kinase assessed as lu... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020253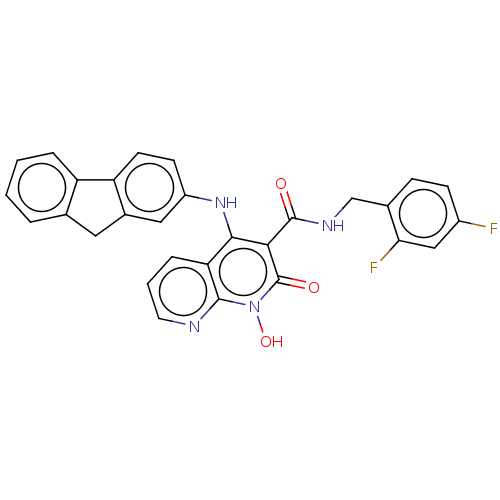 (CHEMBL3288814) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase-mediated viral DNA synthesis in human HOS cells assessed as luciferase activity treated 3 hrs befo... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50061574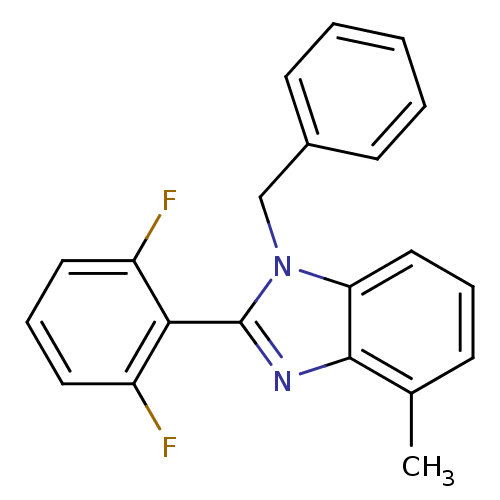 (1-Benzyl-2-(2,6-difluoro-phenyl)-4-methyl-1H-benzo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description The quantity of compound required to reduce WT Reverse transcriptase activity by 50% | J Med Chem 40: 4199-207 (1998) Article DOI: 10.1021/jm970096g BindingDB Entry DOI: 10.7270/Q25B01KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50481091 (CHEMBL27473) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M184V mutant-mediated viral DNA synthesis in human HOS cells assessed as luciferase activity treated 3 hrs b... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020276 (CHEMBL3288825) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020309 (CHEMBL3288837) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50481091 (CHEMBL27473) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase-mediated viral DNA synthesis in human HOS cells assessed as luciferase activity treated 3 hrs befo... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase M184V mutant-mediated viral DNA synthesis in human HOS cells assessed as luciferase activity treated 3 hrs b... | J Med Chem 52: 5356-64 (2009) Article DOI: 10.1021/jm801176e BindingDB Entry DOI: 10.7270/Q29026MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020260 (CHEMBL3288818) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020307 (CHEMBL3288835) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020277 (CHEMBL3288826) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020279 (CHEMBL3288828) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020294 (CHEMBL3288834) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020282 (CHEMBL3288831) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020259 (CHEMBL3288817) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020281 (CHEMBL3288830) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020265 (CHEMBL3288822) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 3'-processing activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |