

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220046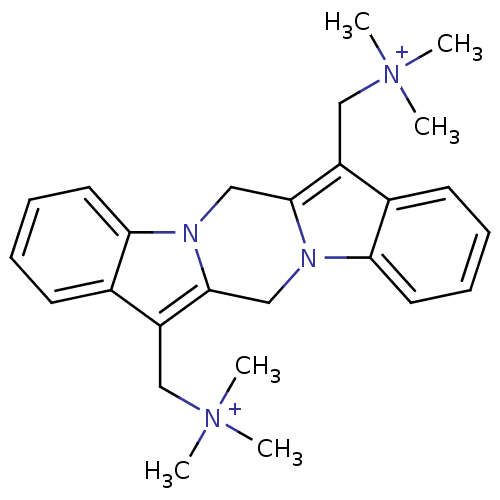 (CHEMBL382243 | trimethyl({20-[(trimethylazaniumyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220045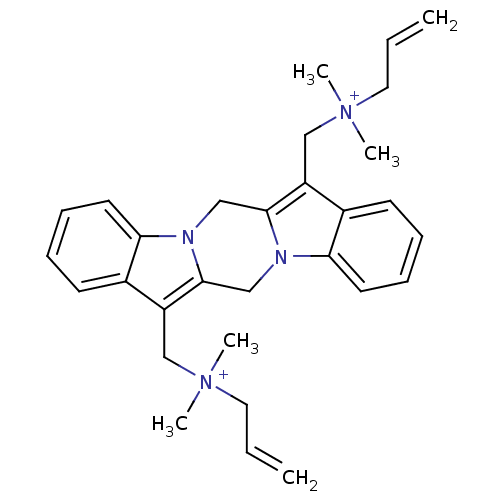 (CHEMBL206666 | [(20-{[dimethyl(prop-2-en-1-yl)azan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50475690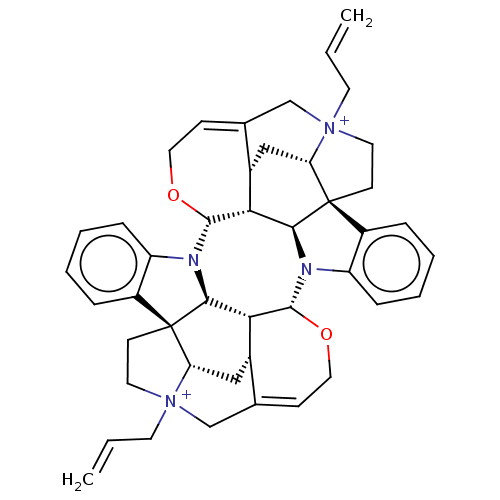 (4,4''-Diallylcaracurinium V Dibromide | Diallylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50475689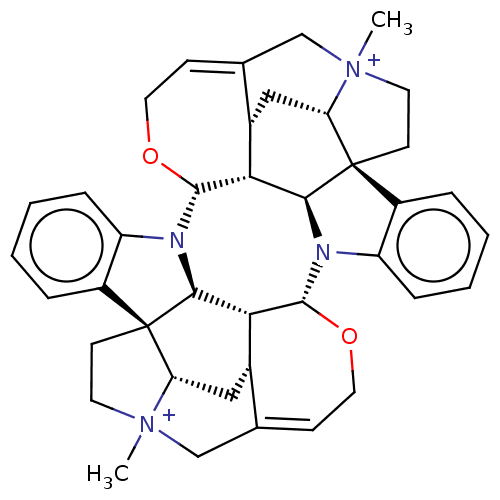 (4,4''-Dimethylcaracurinium V Dichloride | Dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50220060 ((14-dimethylaminomethyl-6H,13H-pyrazino[1,2-a;4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50025037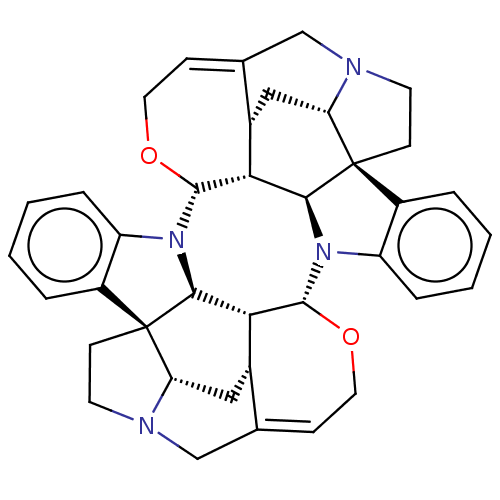 (CHEBI:3382 | Caracurine V) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496212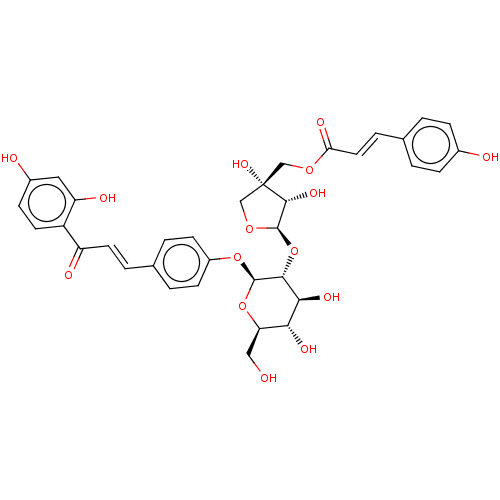 (CHEMBL3125438) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50041517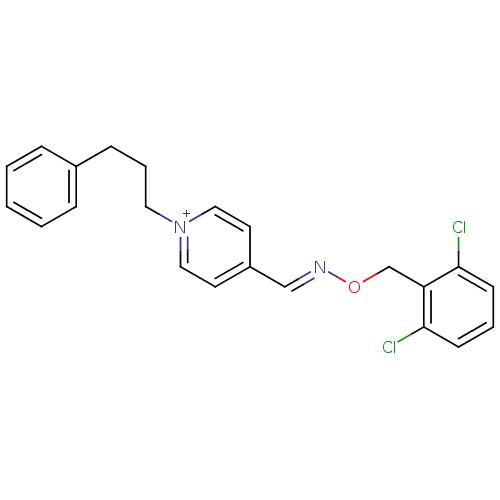 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496209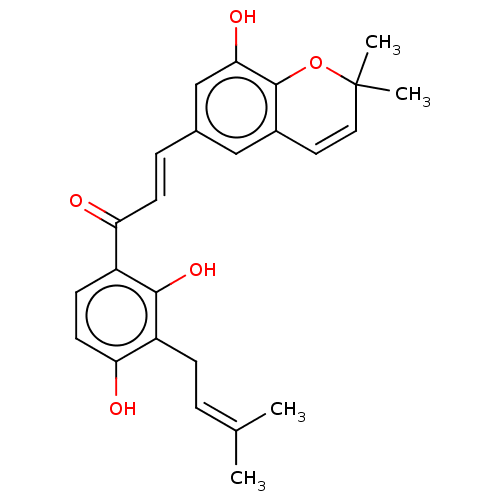 (CHEMBL2204386) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307921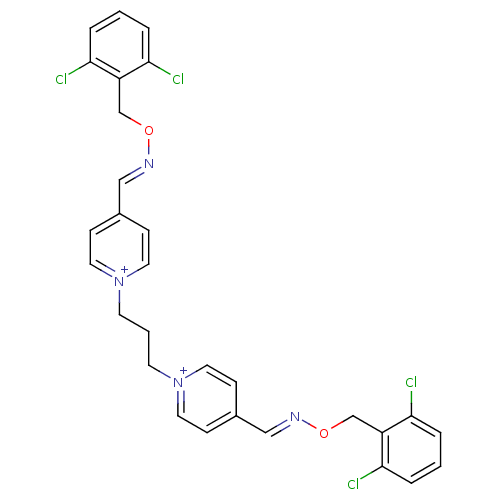 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496207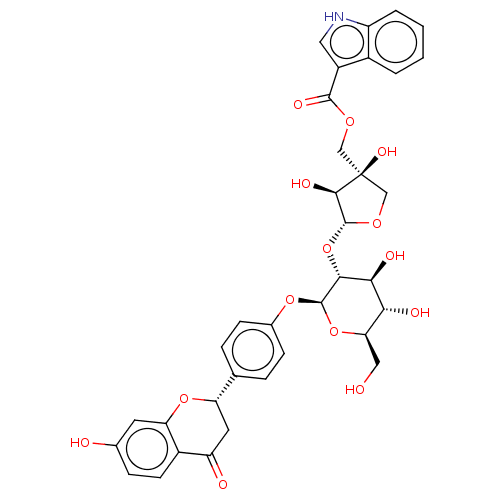 (CHEMBL3125439) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624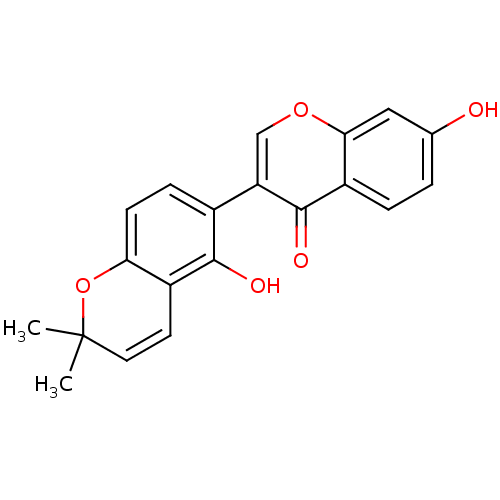 (GLABRONE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM69609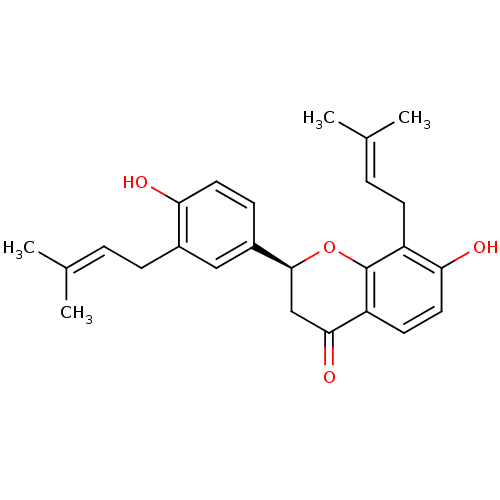 ((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50255625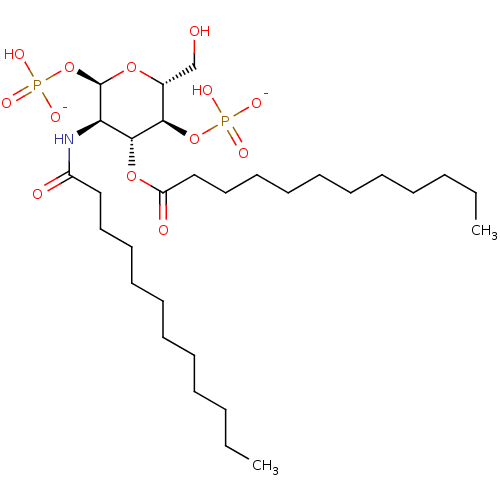 (CHEMBL4070714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology and Biosciences , University of Milano-Bicocca , Piazza della Scienza, 2 , 20126 Milano , Italy. Curated by ChEMBL | Assay Description Antagonist activity at human TLR4 expressed in HEK blue cells assessed as inhibition of LPS-induced NF-kappaB activation-mediated SEAP production pre... | J Med Chem 61: 2895-2909 (2018) Article DOI: 10.1021/acs.jmedchem.7b01803 BindingDB Entry DOI: 10.7270/Q21N83KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496208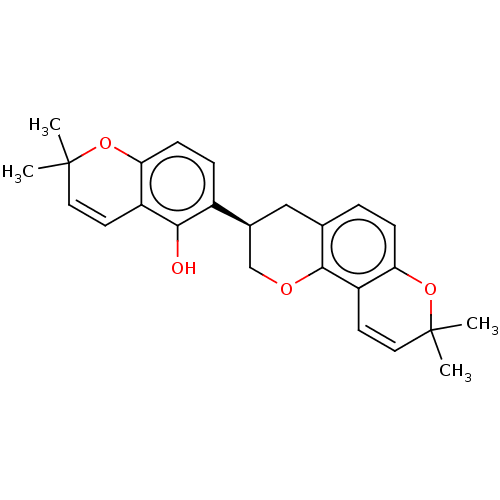 (HISPAGLABRIDIN B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496208 (HISPAGLABRIDIN B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496209 (CHEMBL2204386) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50021398 (CHEBI:18088 | FORMONONETIN | NSC-93360) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50157304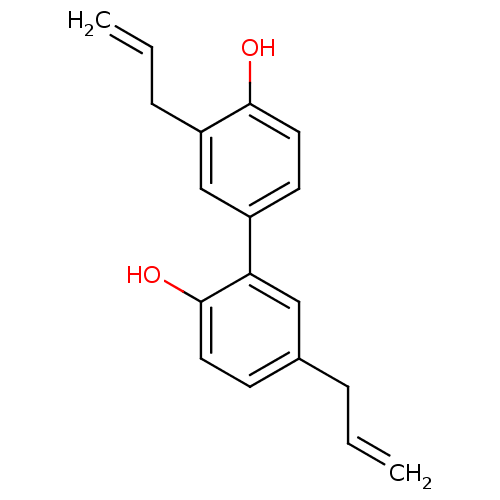 (3',5-di-2-propenyl-1,1'-biphenyl-2,4'-diol | 3',5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50255625 (CHEMBL4070714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology and Biosciences , University of Milano-Bicocca , Piazza della Scienza, 2 , 20126 Milano , Italy. Curated by ChEMBL | Assay Description Antagonist activity at human TLR4 expressed in mouse RAW blue cells assessed as inhibition of LPS-induced NF-kappaB activation-mediated SEAP producti... | J Med Chem 61: 2895-2909 (2018) Article DOI: 10.1021/acs.jmedchem.7b01803 BindingDB Entry DOI: 10.7270/Q21N83KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307921 ((E,E)-1,3-bis[4-[[(2,6-dichlorobenzyloxyl)imino]me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50255632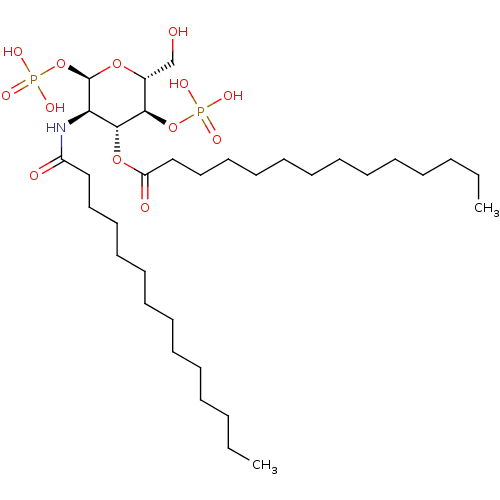 (CHEMBL4100044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology and Biosciences , University of Milano-Bicocca , Piazza della Scienza, 2 , 20126 Milano , Italy. Curated by ChEMBL | Assay Description Antagonist activity at human TLR4 expressed in HEK blue cells assessed as inhibition of LPS-induced NF-kappaB activation-mediated SEAP production pre... | J Med Chem 61: 2895-2909 (2018) Article DOI: 10.1021/acs.jmedchem.7b01803 BindingDB Entry DOI: 10.7270/Q21N83KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50241408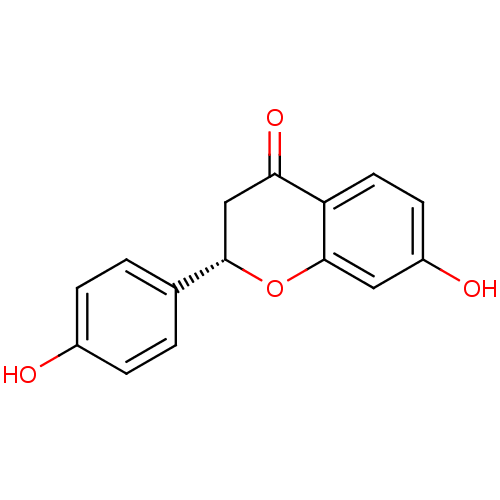 ((2S)-liquiritigenin | 7-HYDROXY-2-(4-HYDROXY-PHENY...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496208 (HISPAGLABRIDIN B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50041517 (4-[(2,6-dichloro-benzyloxyimino)-methyl]-1-(3-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496210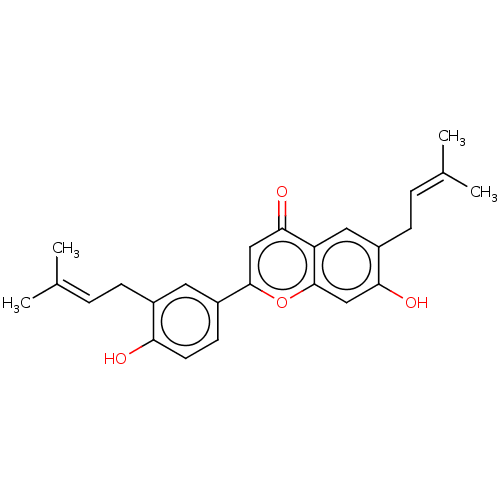 (CHEMBL3125437) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496212 (CHEMBL3125438) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496208 (HISPAGLABRIDIN B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50249470 (CHEBI:28327 | Prunin) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM69609 ((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50021398 (CHEBI:18088 | FORMONONETIN | NSC-93360) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM69609 ((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496207 (CHEMBL3125439) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50249470 (CHEBI:28327 | Prunin) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50021398 (CHEBI:18088 | FORMONONETIN | NSC-93360) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50021398 (CHEBI:18088 | FORMONONETIN | NSC-93360) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50255626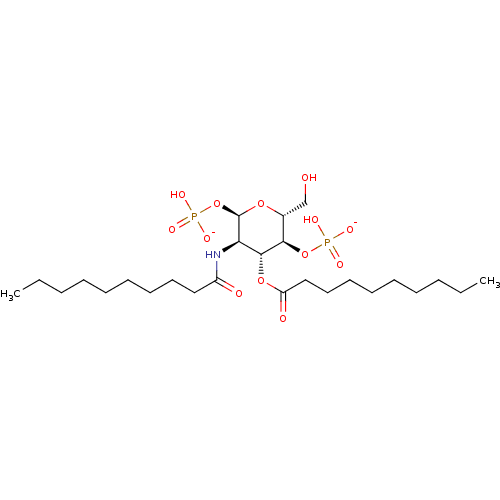 (CHEMBL4069693) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology and Biosciences , University of Milano-Bicocca , Piazza della Scienza, 2 , 20126 Milano , Italy. Curated by ChEMBL | Assay Description Antagonist activity at human TLR4 expressed in HEK blue cells assessed as inhibition of LPS-induced NF-kappaB activation-mediated SEAP production pre... | J Med Chem 61: 2895-2909 (2018) Article DOI: 10.1021/acs.jmedchem.7b01803 BindingDB Entry DOI: 10.7270/Q21N83KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307927 (4-((2,6-dichlorobenzylidene)hydrazono)-1-(3-phenyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50307928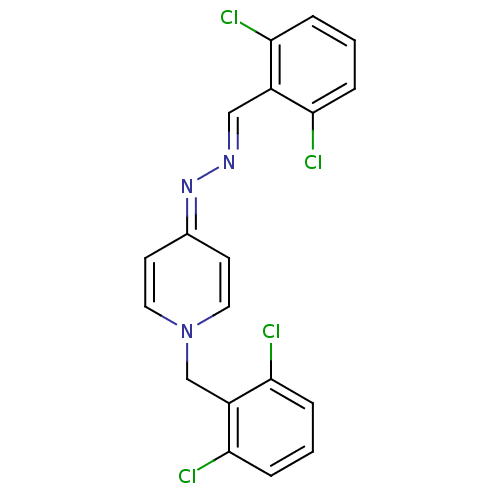 (1-(2,6-dichlorobenzyl)-4-((2,6-dichlorobenzylidene...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50496211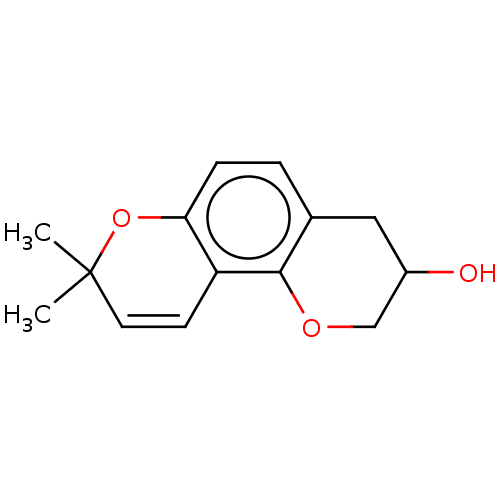 (CHEMBL3125436) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50255632 (CHEMBL4100044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biotechnology and Biosciences , University of Milano-Bicocca , Piazza della Scienza, 2 , 20126 Milano , Italy. Curated by ChEMBL | Assay Description Antagonist activity at human TLR4 expressed in mouse RAW blue cells assessed as inhibition of LPS-induced NF-kappaB activation-mediated SEAP producti... | J Med Chem 61: 2895-2909 (2018) Article DOI: 10.1021/acs.jmedchem.7b01803 BindingDB Entry DOI: 10.7270/Q21N83KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307925 (1-benzyl-4-((2,6-dichlorobenzylidene)hydrazono)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50241408 ((2S)-liquiritigenin | 7-HYDROXY-2-(4-HYDROXY-PHENY...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307926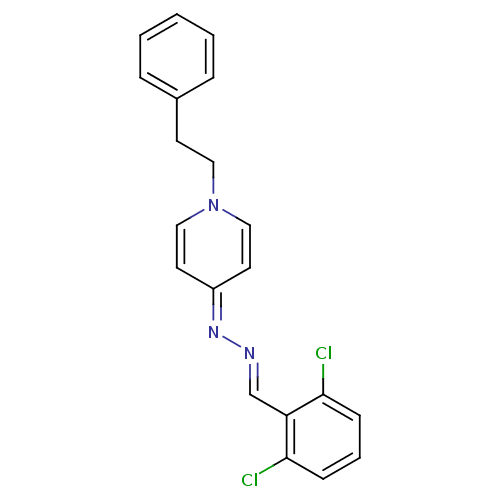 (4-((2,6-dichlorobenzylidene)hydrazono)-1-phenethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege-University Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by modified Ellman's method | Bioorg Med Chem 18: 2049-59 (2010) Article DOI: 10.1016/j.bmc.2010.01.002 BindingDB Entry DOI: 10.7270/Q2GH9J3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |