

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345099 (4-[(2-{5-[5-chloro-2-(1H- tetrazol-1-yl)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345082 (4-{[(2R)-2-{5-[5-chloro-2- (1H-tetrazol-1-yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345057 (4-{[(2R)-2-{5-[5-chloro- 2-(1H-tetrazol-1- yl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50371255 (CHEMBL1203953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345105 (4-[(2-{5-[3-chloro-2- fluoro-6-(1H-tetrazol-1- yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345061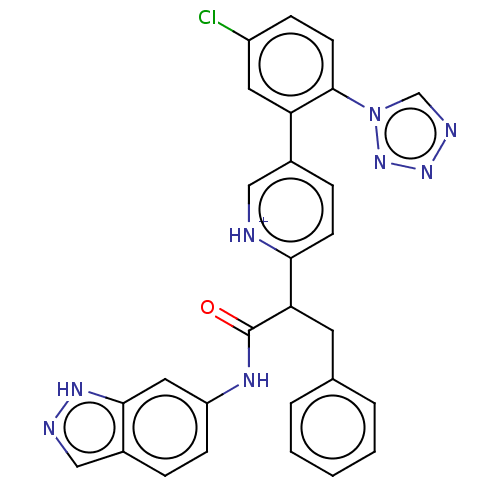 (2-{5-[5-chloro-2-(1H- tetrazol-1-yl)phenyl]-1- oxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345063 (4-{[(2R)-2-{5-[5-chloro- 2-(1H-tetrazol-1- yl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345078 (2-(1-((4-Carboxyphenyl)amino)-1-oxo-3-phenylpropan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232520 ((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232520 ((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232506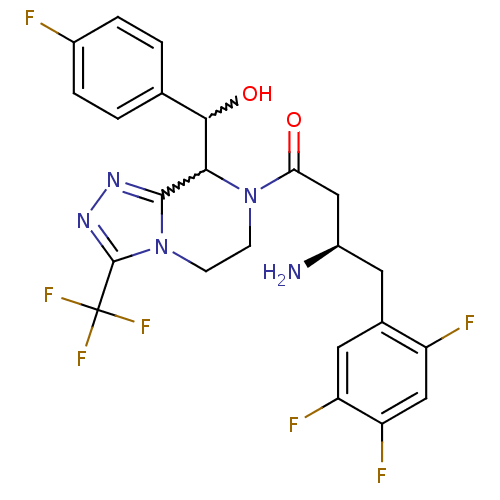 (2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232518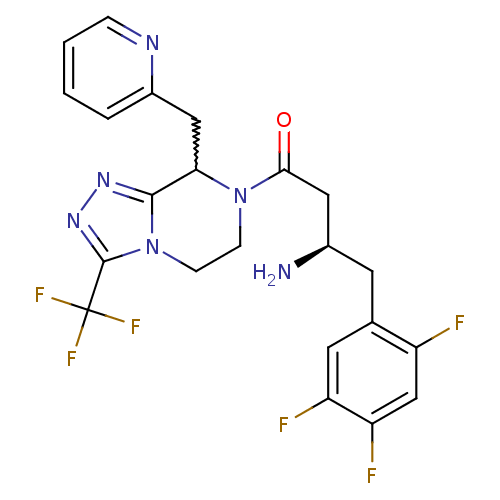 ((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232518 ((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232503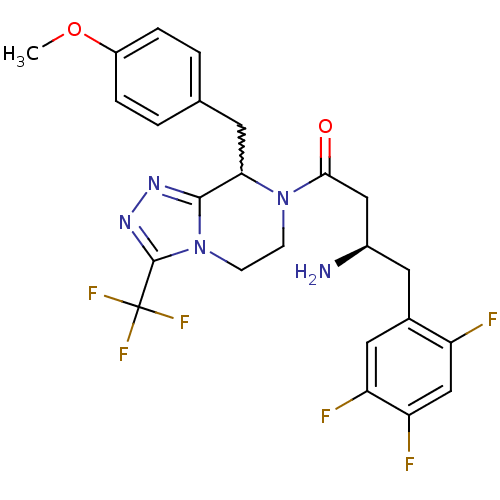 ((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232503 ((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232501 ((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232501 ((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345103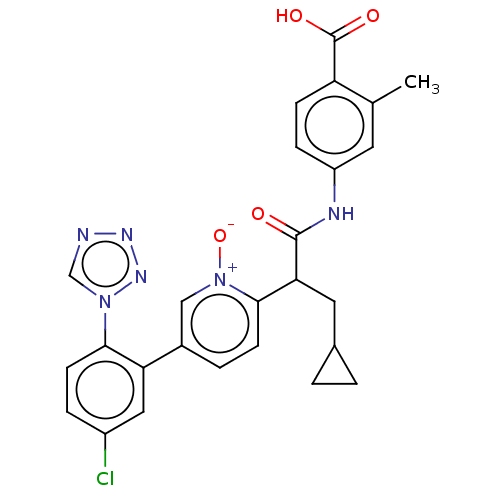 (4-[(2-{5-[5-chloro-2-(1H- tetrazol-1-yl)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232506 (2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232506 (2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345076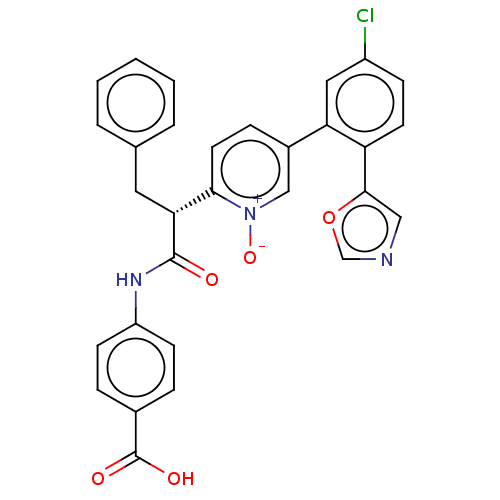 (4-{[(2R)-2-{5-[5-chloro- 2-(1,3-oxazol-5- yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232510 ((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50232510 ((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 | J Med Chem 51: 589-602 (2008) Article DOI: 10.1021/jm070330v BindingDB Entry DOI: 10.7270/Q2B27W4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345096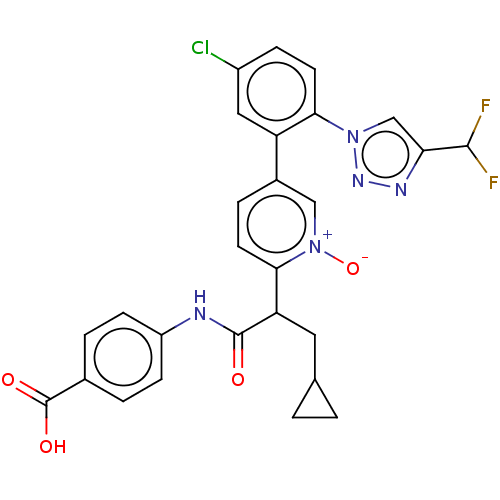 (4-{[2-(5-{5-chloro-2-[4- (difluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM345078 (2-(1-((4-Carboxyphenyl)amino)-1-oxo-3-phenylpropan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Kallikrein determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; Fishe... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345072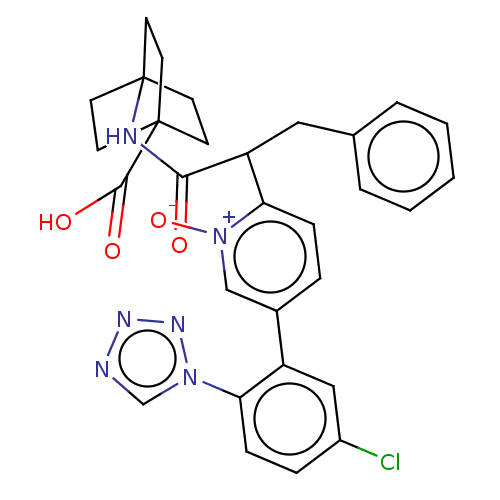 (4-[(2-{5-[5-chloro-2- (1H-tetrazol-1- yl)phenyl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204715 (US9243002, 55) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.971 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204706 (US9243002, 50 | US9243002, 51) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM345057 (4-{[(2R)-2-{5-[5-chloro- 2-(1H-tetrazol-1- yl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Kallikrein determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; Fishe... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204785 (US9243002, 83 | US9243002, 86) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204721 (US9243002, 58 | US9243002, 59) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204712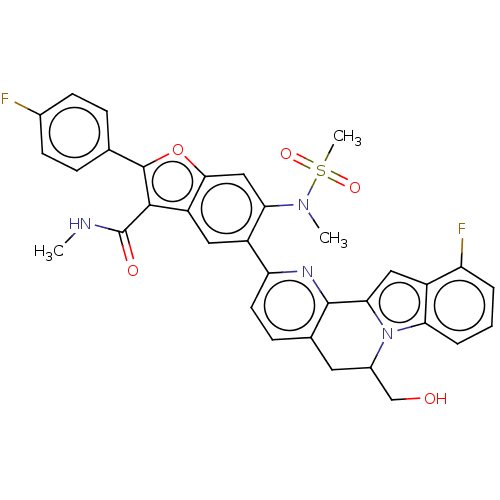 (US9243002, 52 | US9243002, 53 | US9243002, 54) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204721 (US9243002, 58 | US9243002, 59) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204697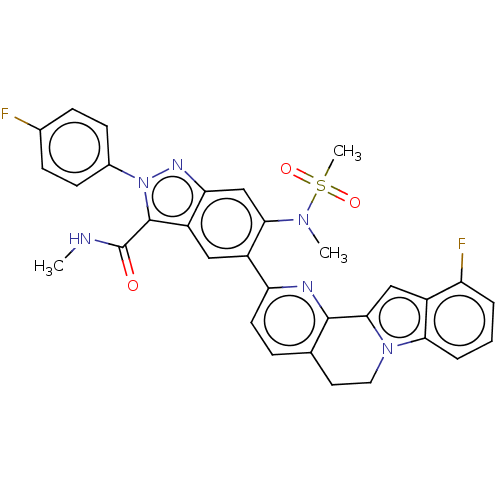 (US9243002, 48) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204662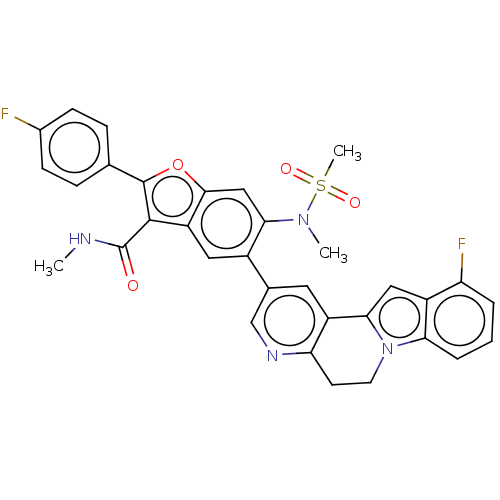 (US9243002, 43) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204669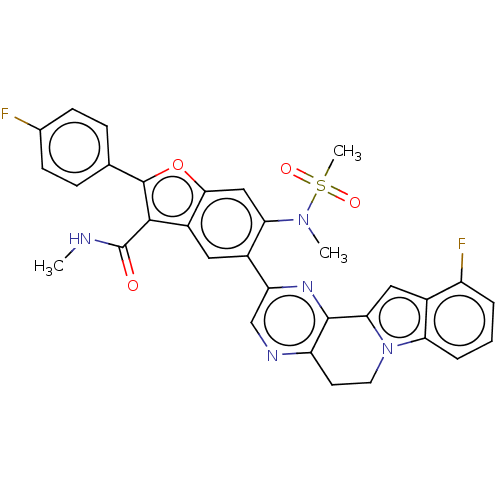 (US9243002, 44) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204715 (US9243002, 55) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204669 (US9243002, 44) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM345061 (2-{5-[5-chloro-2-(1H- tetrazol-1-yl)phenyl]-1- oxi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Kallikrein determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; Fishe... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204785 (US9243002, 83 | US9243002, 86) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204716 (US9243002, 56 | US9243002, 57) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204760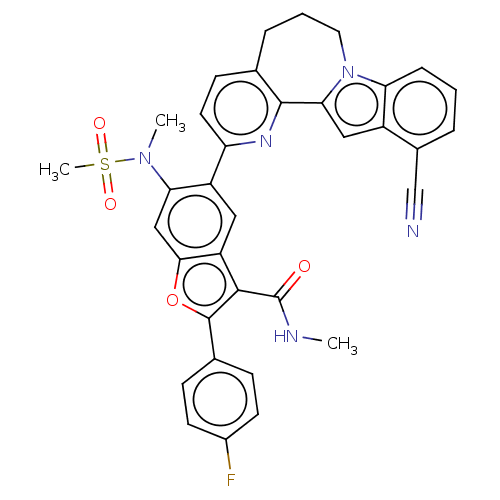 (US9243002, 73 | US9243002, 75) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204583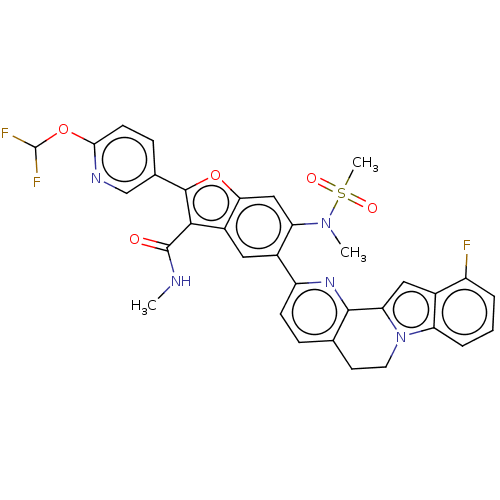 (US9243002, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM345096 (4-{[2-(5-{5-chloro-2-[4- (difluoromethyl)-1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9783530 (2017) BindingDB Entry DOI: 10.7270/Q2N87CXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204801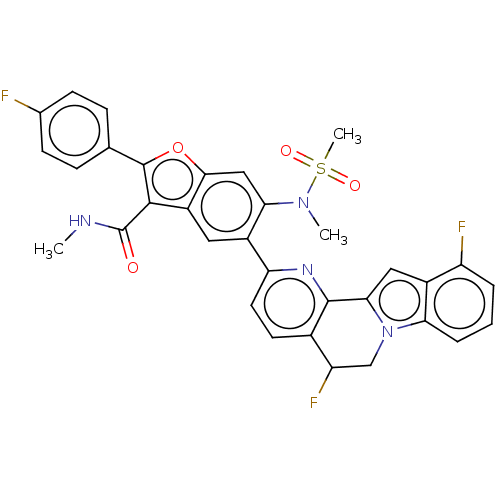 (US9243002, 89) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204677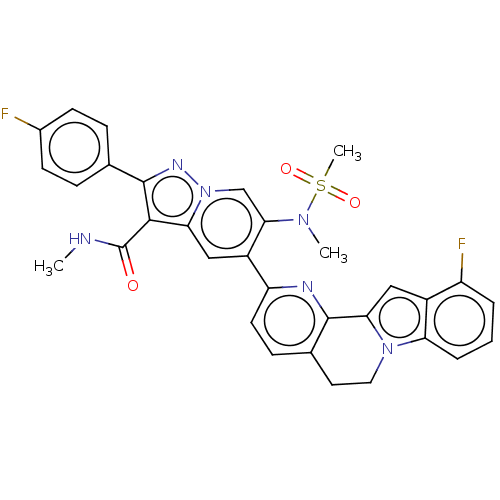 (US9243002, 46 | US9243002, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204712 (US9243002, 52 | US9243002, 53 | US9243002, 54) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204723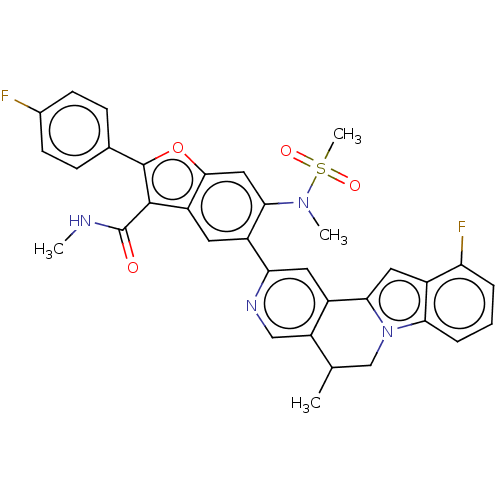 (US9243002, 60 | US9243002, 61) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM204750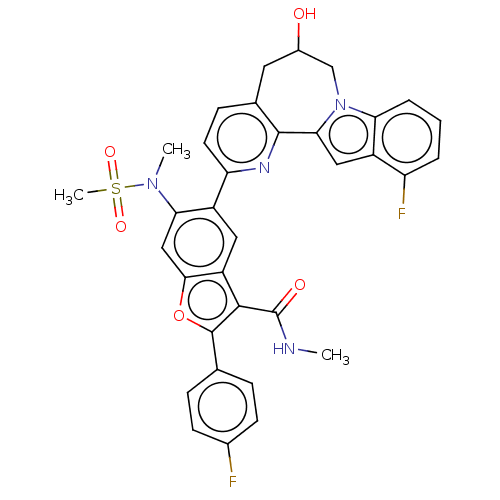 (US9243002, 69) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM204586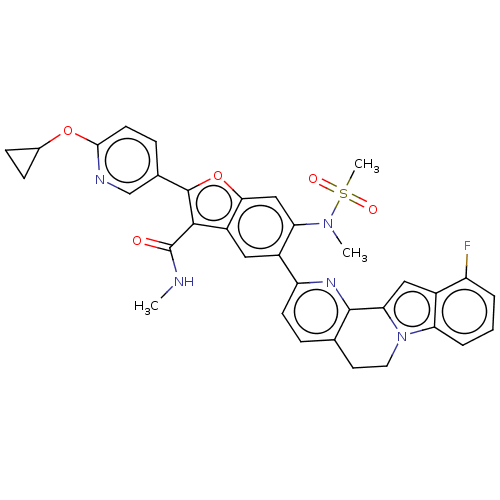 (US9243002, 5) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To initiate an assay, replicon cells were plated in the presence of a dilution series of test compound in the absence of G418. Typically, the assays ... | US Patent US9243002 (2016) BindingDB Entry DOI: 10.7270/Q2P55MBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 819 total ) | Next | Last >> |