

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]RTI55 binding from human wild type SERT | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113533 BindingDB Entry DOI: 10.7270/Q24T6P6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50307501 ((6S)-N,N-dipropyl-5,6,7,8-tetrahydro-1,1'-binaphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29563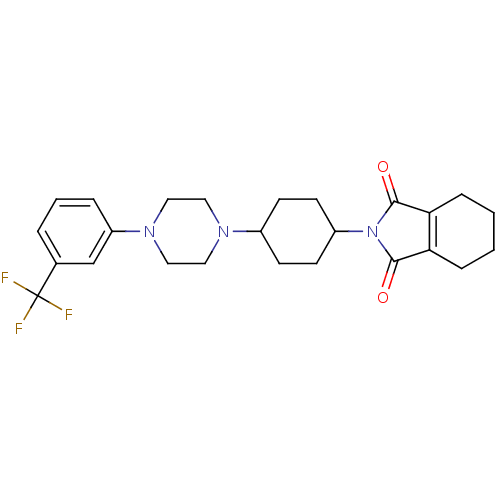 (1-(m-trifluorophenyl)piperazine, 10) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50139351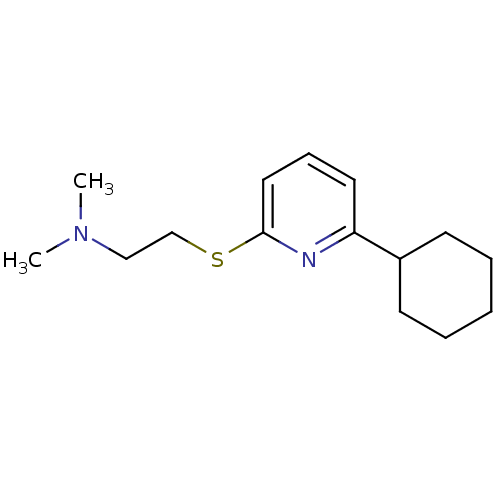 (2-(6-cyclohexylpyridin-2-ylthio)-N,N-dimethylethan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50419052 (SB-399885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... | Eur J Med Chem 170: 261-275 (2019) Article DOI: 10.1016/j.ejmech.2019.03.017 BindingDB Entry DOI: 10.7270/Q2222Z6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-methylspiperone from human D2 long receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT3 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562710 (CHEMBL4759730) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112916 BindingDB Entry DOI: 10.7270/Q22R3WD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50578580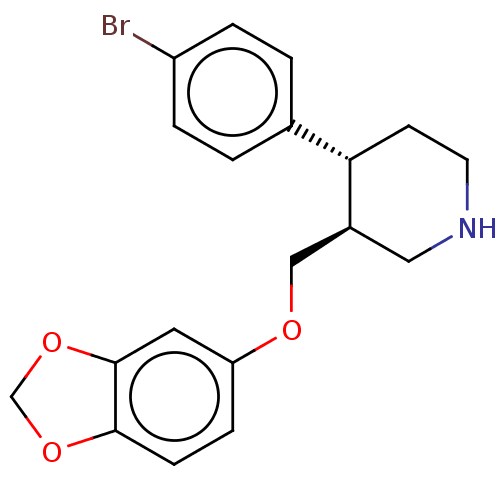 (CHEMBL4848081) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]RTI55 binding from human wild type SERT | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113533 BindingDB Entry DOI: 10.7270/Q24T6P6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29567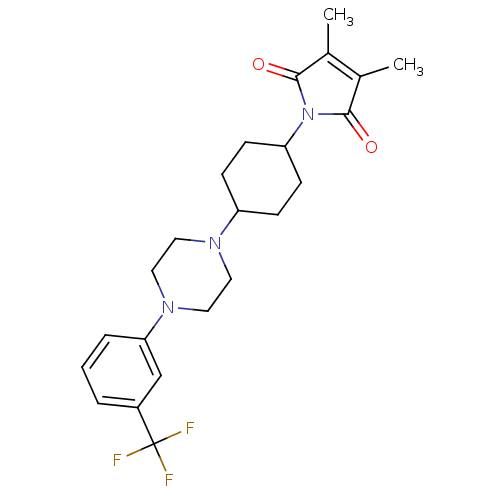 (1-(m-trifluorophenyl)piperazine, 14) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29565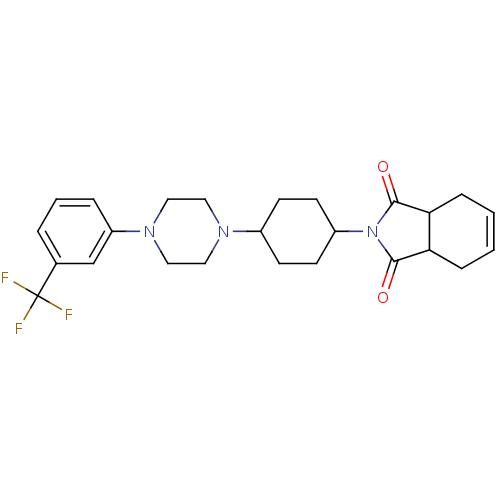 (1-(m-trifluorophenyl)piperazine, 12) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513415 (CHEMBL4435010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50504839 (CHEMBL4584504) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50504838 (CHEMBL4437523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579342 (CHEMBL4872586) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579341 (CHEMBL4860809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579339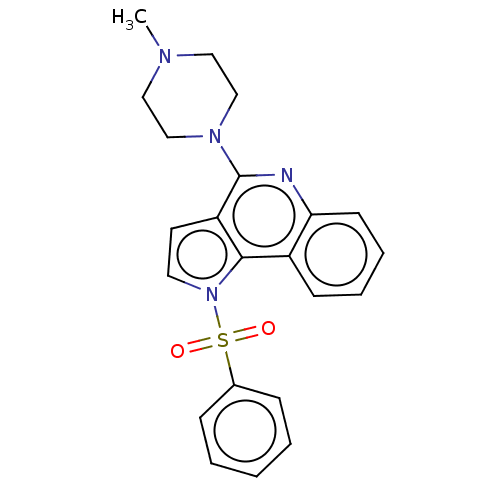 (CHEMBL4865309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29566 (1-(m-trifluorophenyl)piperazine, 13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150684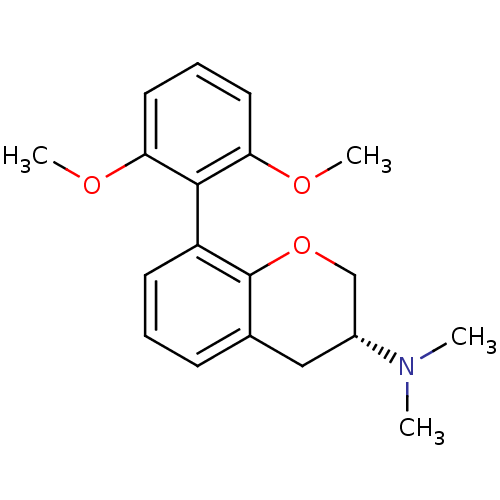 ((R)-8-(2,6-dimethoxyphenyl)-N,N-dimethylchroman-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29561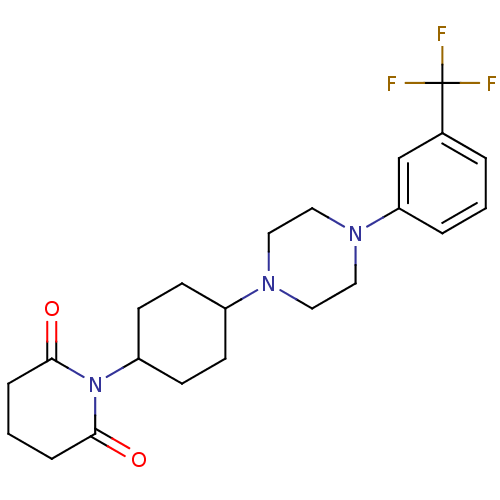 (1-(m-trifluorophenyl)piperazine, 8) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150685 (((S)-5-Phenyl-1,2,3,4-tetrahydro-naphthalen-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579349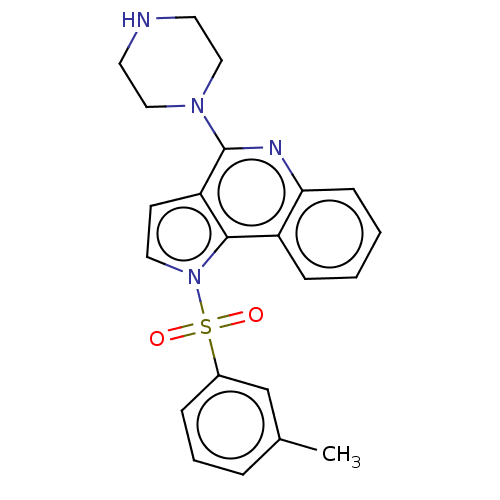 (CHEMBL4870374) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579348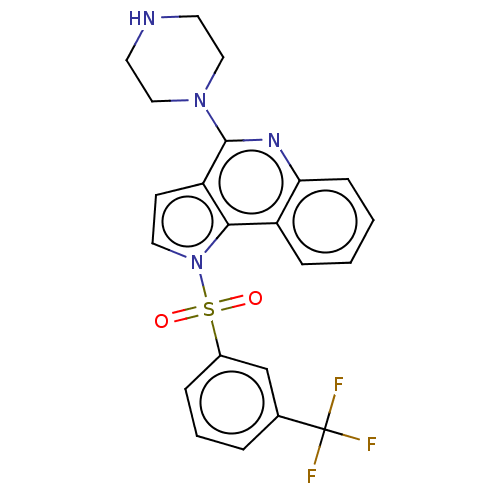 (CHEMBL4862354) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579347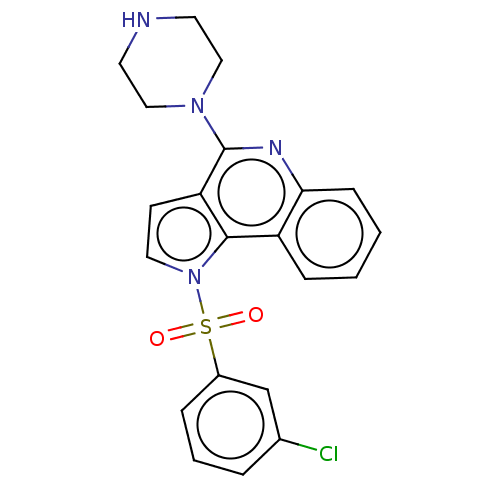 (CHEMBL4846153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50274767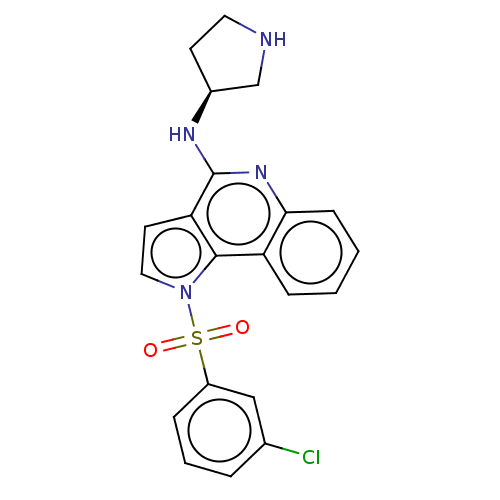 (CHEMBL4125735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50504842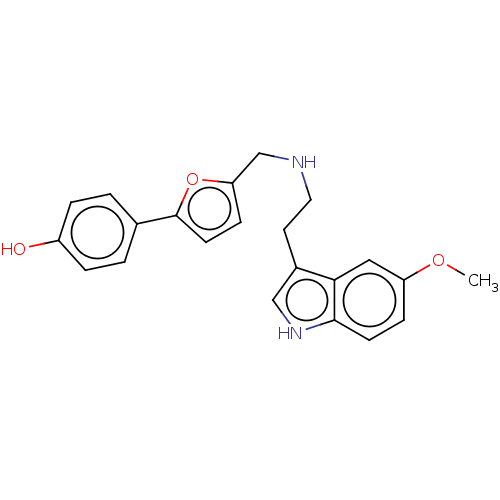 (CHEMBL4583082) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29560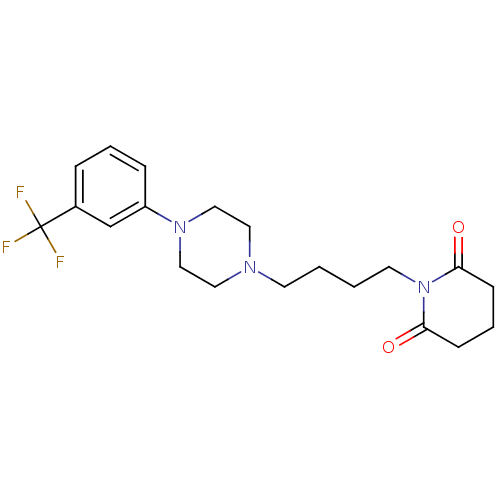 (1-(m-trifluorophenyl)piperazine, 7) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513432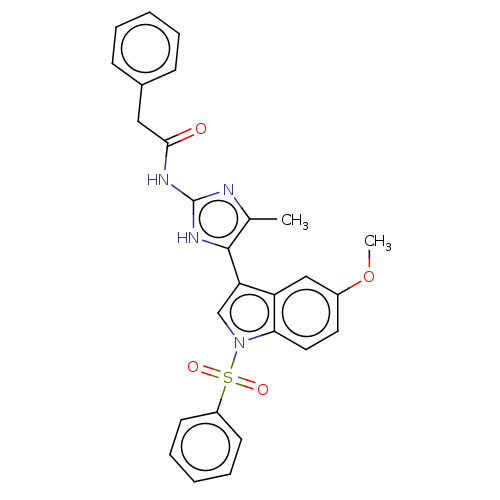 (CHEMBL4586990) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513438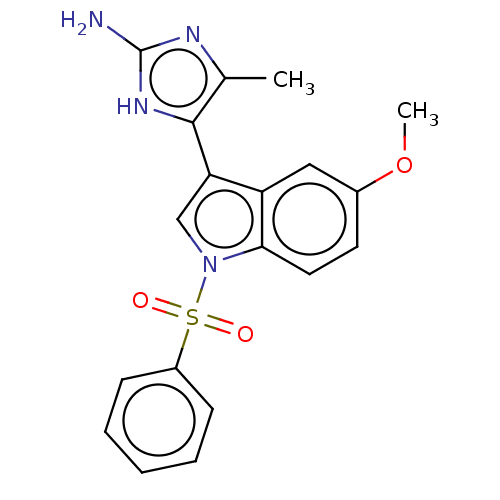 (CHEMBL4574931) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150676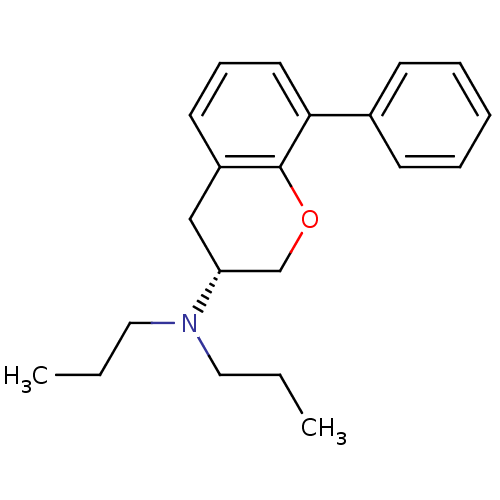 (((R)-8-Phenyl-chroman-3-yl)-dipropyl-amine | (R)-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50019754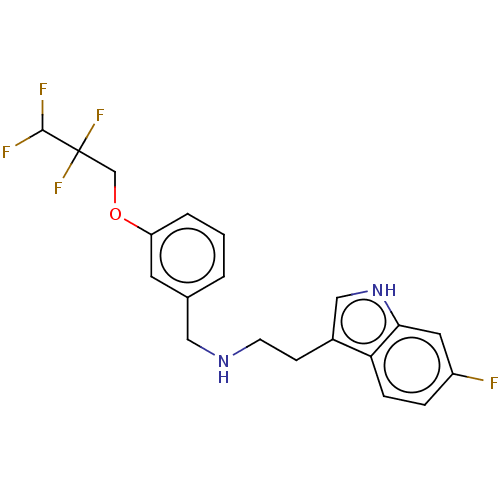 (IDALOPIRDINE | LU-AE58054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29557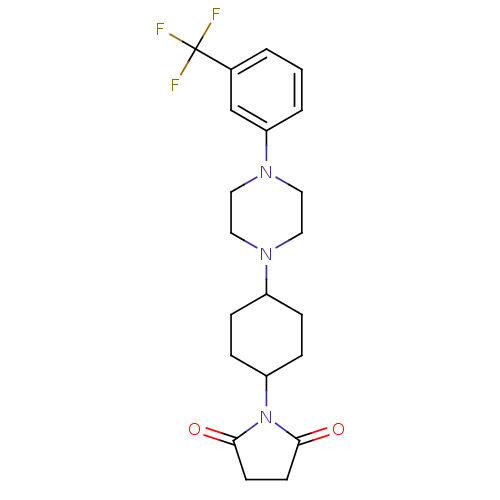 (1-(m-trifluorophenyl)piperazine, 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM29558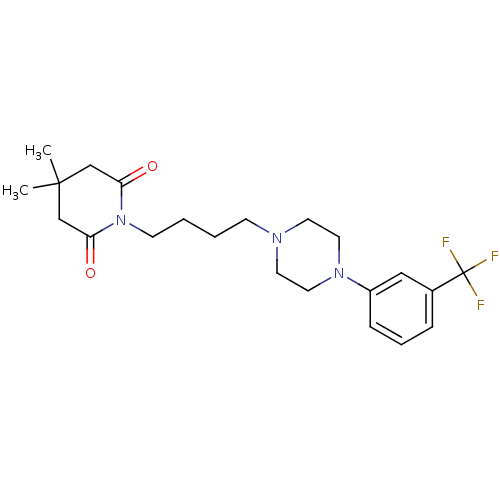 (1-(m-trifluorophenyl)piperazine, 5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50513435 (CHEMBL4472629) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells measured after 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50524111 (CHEMBL4452569) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... | Eur J Med Chem 170: 261-275 (2019) Article DOI: 10.1016/j.ejmech.2019.03.017 BindingDB Entry DOI: 10.7270/Q2222Z6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579356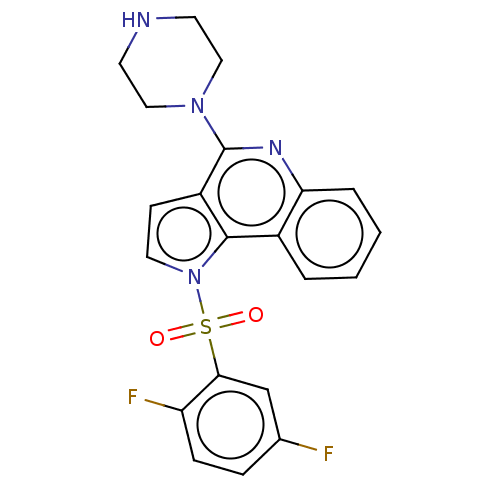 (CHEMBL4853195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM29563 (1-(m-trifluorophenyl)piperazine, 10) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Science | Assay Description Competition experiments were performed in the presence radioligand with membrane protein and test compounds. After incubation, the reaction stopped b... | Bioorg Med Chem 15: 7116-25 (2007) Article DOI: 10.1016/j.bmc.2007.07.029 BindingDB Entry DOI: 10.7270/Q2G15Z6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334252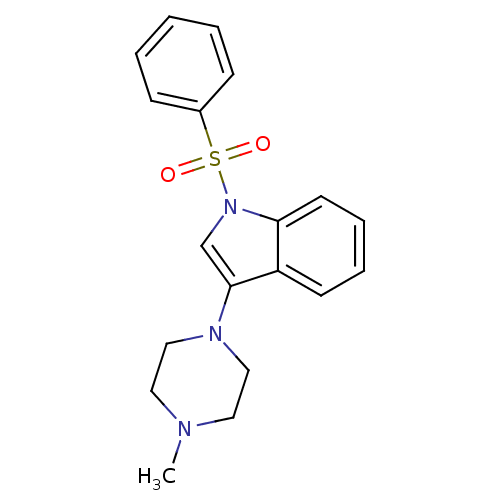 (3-(4-methylpiperazin-1-yl)-1-(phenylsulfonyl)-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor incubated for 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112916 BindingDB Entry DOI: 10.7270/Q22R3WD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50578573 (CHEMBL4869180) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113533 BindingDB Entry DOI: 10.7270/Q24T6P6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579345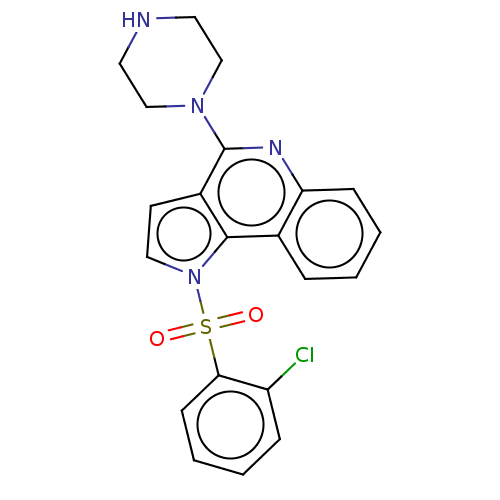 (CHEMBL4868468) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50524116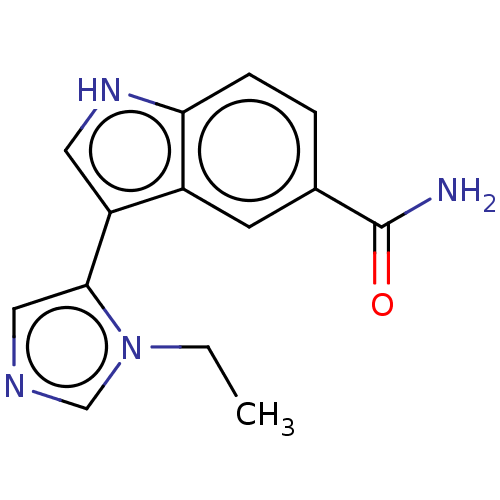 (CHEMBL4469847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... | Eur J Med Chem 170: 261-275 (2019) Article DOI: 10.1016/j.ejmech.2019.03.017 BindingDB Entry DOI: 10.7270/Q2222Z6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50504843 (CHEMBL4438434) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in HEK293 cells after 1 hr by microbeta plate reader analysis | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111857 BindingDB Entry DOI: 10.7270/Q2833W92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells incubated for 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHOK1 cells incubated for 1 hr by liquid scintillation counter method | Eur J Med Chem 179: 1-15 (2019) Article DOI: 10.1016/j.ejmech.2019.06.001 BindingDB Entry DOI: 10.7270/Q2S185TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35254 (2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor expressed in CHOK1 cells after 1 hr by microbeta scintillation counting method | Eur J Med Chem 170: 261-275 (2019) Article DOI: 10.1016/j.ejmech.2019.03.017 BindingDB Entry DOI: 10.7270/Q2222Z6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 705 total ) | Next | Last >> |