

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-2 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-3 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097248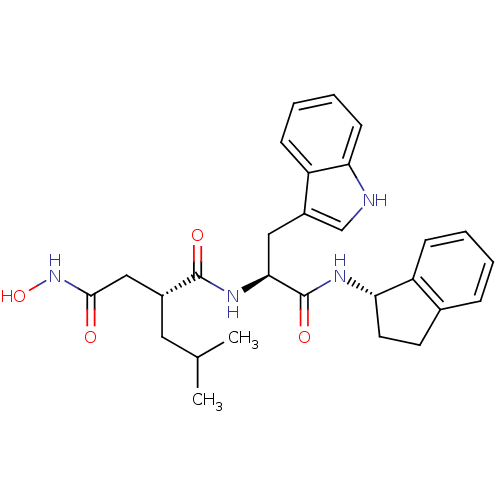 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-indan-1-ylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-2 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097248 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-indan-1-ylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-3 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097257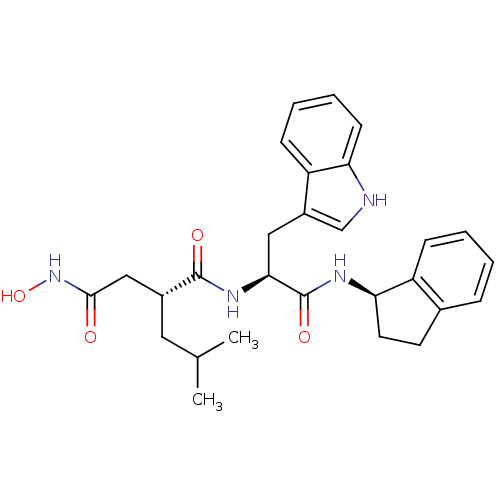 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((R)-indan-1-ylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-2 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097257 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((R)-indan-1-ylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-3 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097256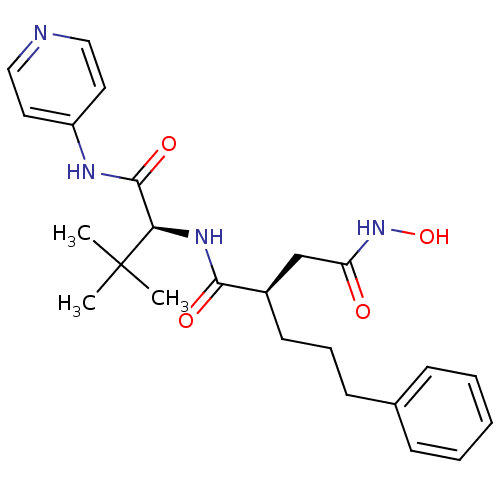 ((R)-N*1*-[(S)-2,2-Dimethyl-1-(pyridin-4-ylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063910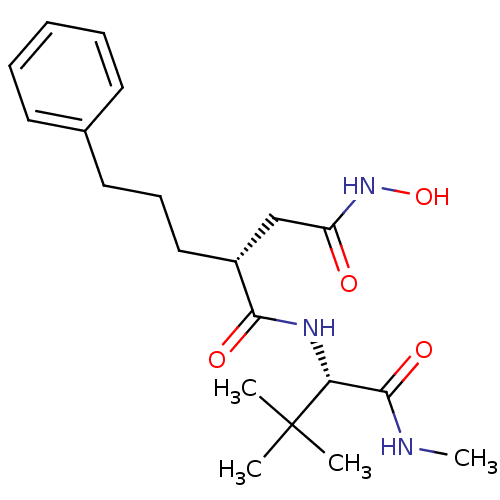 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097249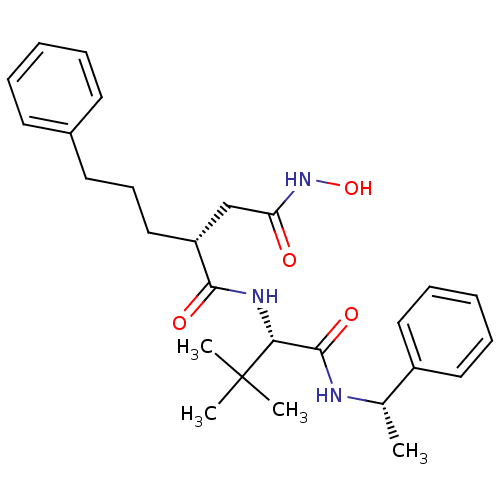 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((S)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-9 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-1 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097245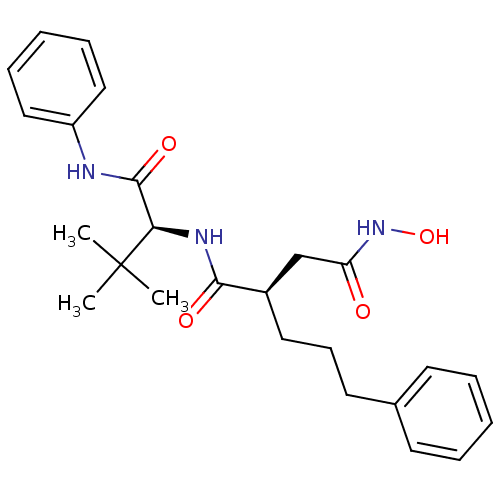 ((R)-N*1*-((S)-2,2-Dimethyl-1-phenylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097240 ((R)-N*1*-((S)-1-Cyclohexylcarbamoyl-2,2-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097254 ((R)-N*1*-((S)-1-Cyclopentylcarbamoyl-2,2-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-14 | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097232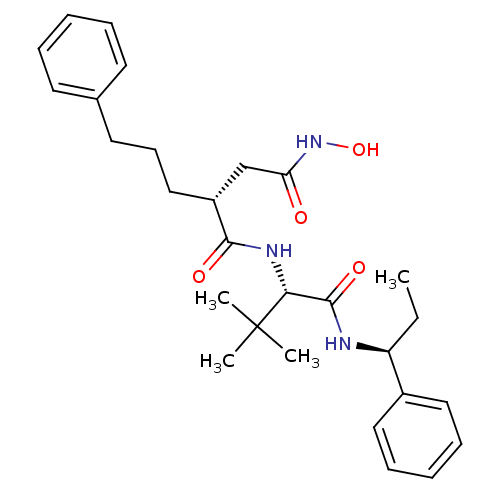 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((S)-1-phenyl-propylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097235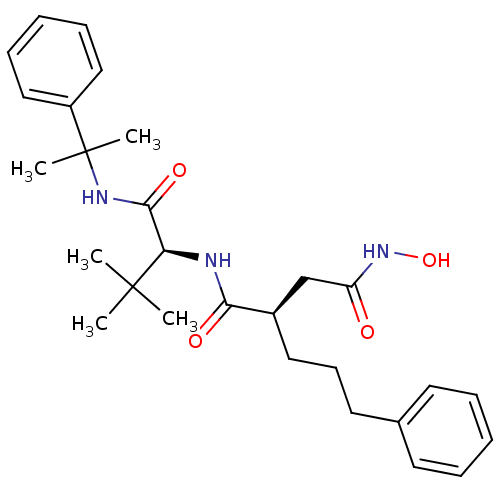 ((R)-N*1*-[(S)-2,2-Dimethyl-1-(1-methyl-1-phenyl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097256 ((R)-N*1*-[(S)-2,2-Dimethyl-1-(pyridin-4-ylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097242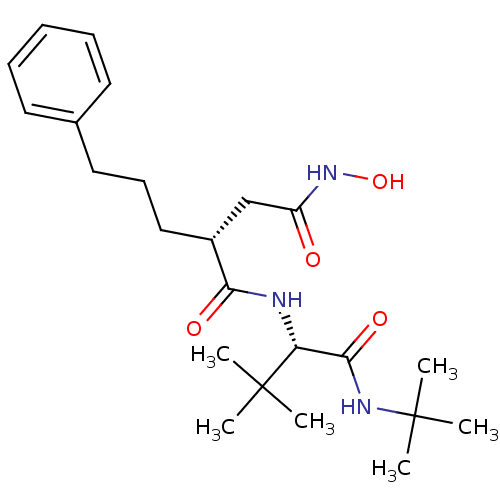 ((R)-N*1*-((S)-1-tert-Butylcarbamoyl-2,2-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097234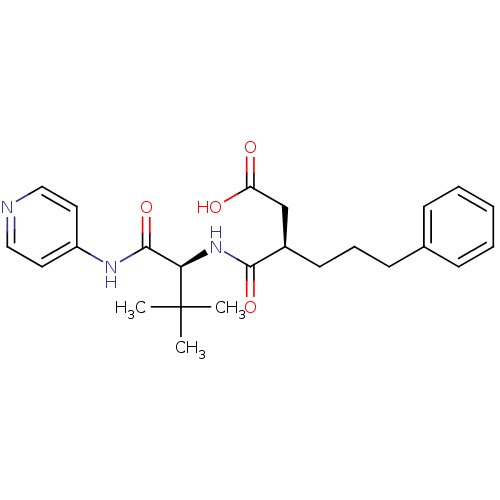 ((R)-3-(((S)-3,3-dimethyl-1-oxo-1-(pyridin-4-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097254 ((R)-N*1*-((S)-1-Cyclopentylcarbamoyl-2,2-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097249 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((S)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097241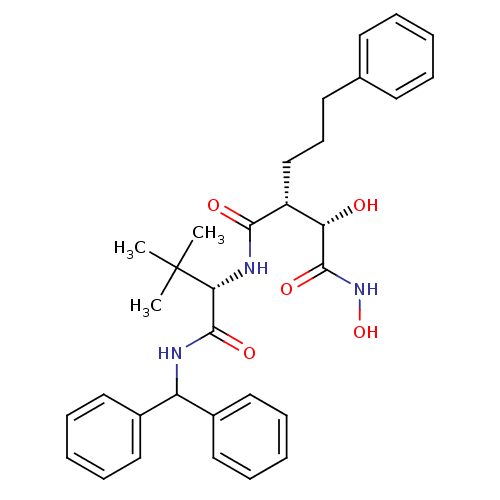 ((2R,3S)-N*4*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097246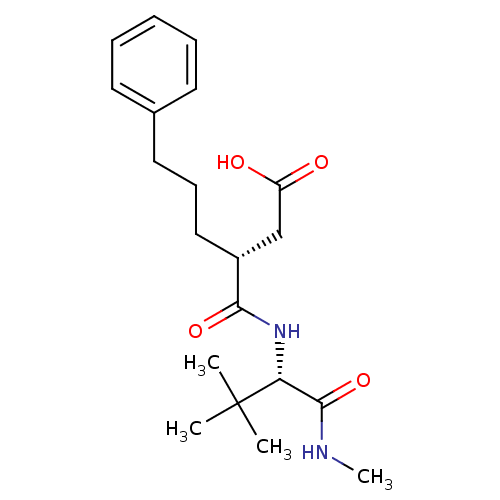 ((R)-3-(((S)-3,3-dimethyl-1-(methylamino)-1-oxobuta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097245 ((R)-N*1*-((S)-2,2-Dimethyl-1-phenylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097238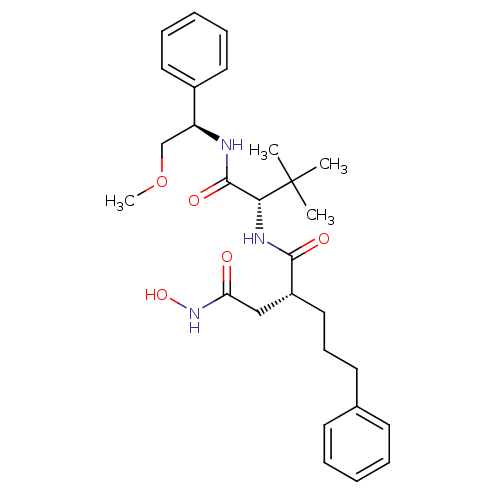 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((R)-2-methoxy-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097232 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((S)-1-phenyl-propylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097240 ((R)-N*1*-((S)-1-Cyclohexylcarbamoyl-2,2-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097242 ((R)-N*1*-((S)-1-tert-Butylcarbamoyl-2,2-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097238 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((R)-2-methoxy-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097231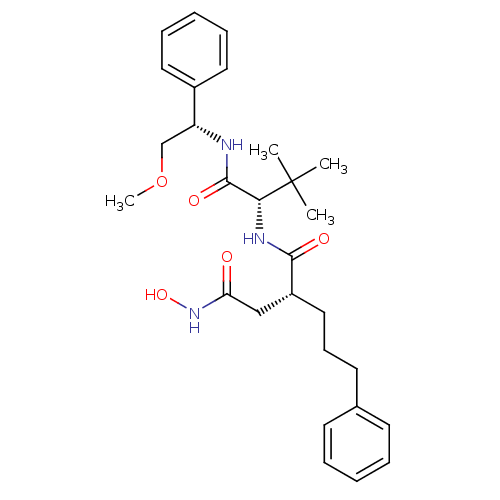 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-methoxy-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097229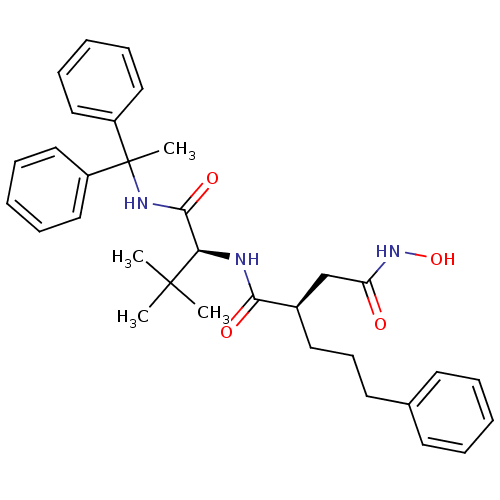 ((R)-N*1*-[(S)-1-(1,1-Diphenyl-ethylcarbamoyl)-2,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097228 ((R)-3-(((S)-3,3-dimethyl-1-oxo-1-(phenylamino)buta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097250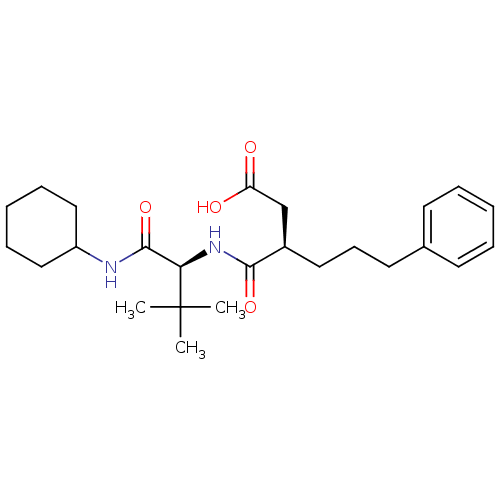 ((R)-3-(((S)-1-(cyclohexylamino)-3,3-dimethyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097252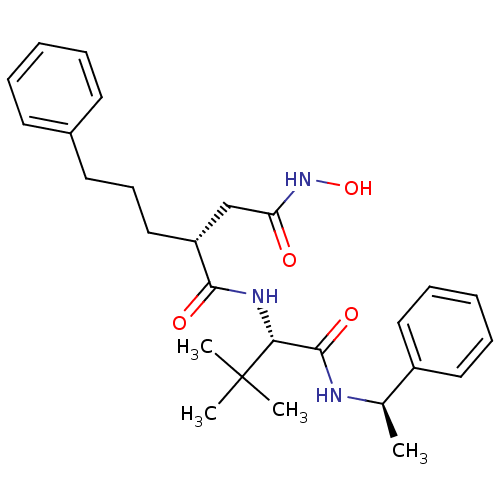 ((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097243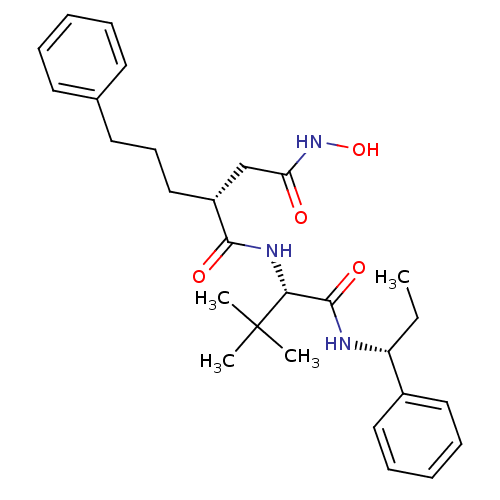 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-propylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097239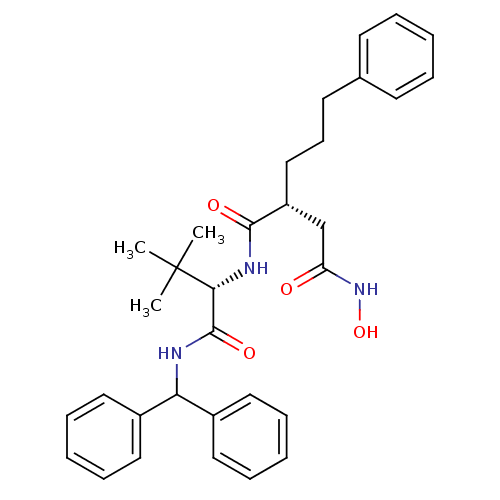 ((R)-N*1*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097253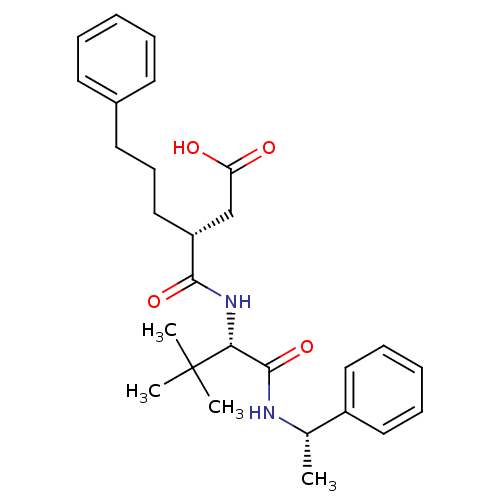 ((R)-3-(((S)-3,3-dimethyl-1-oxo-1-((S)-1-phenylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097227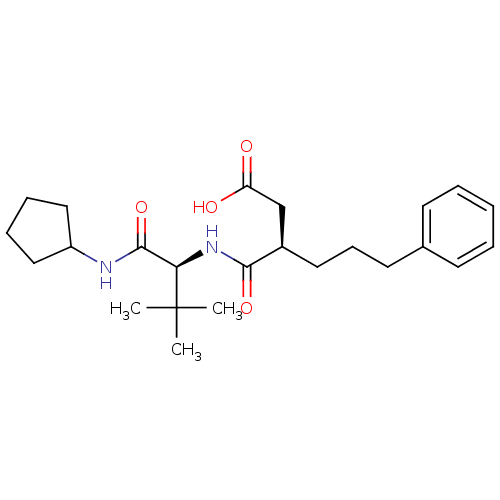 ((R)-3-(((S)-1-(cyclopentylamino)-3,3-dimethyl-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097252 ((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097243 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-propylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097235 ((R)-N*1*-[(S)-2,2-Dimethyl-1-(1-methyl-1-phenyl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097237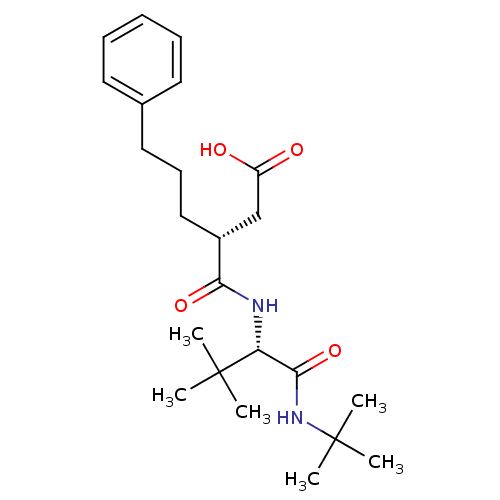 ((R)-3-(((S)-1-(tert-butylamino)-3,3-dimethyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097231 ((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-methoxy-1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097241 ((2R,3S)-N*4*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097234 ((R)-3-(((S)-3,3-dimethyl-1-oxo-1-(pyridin-4-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097251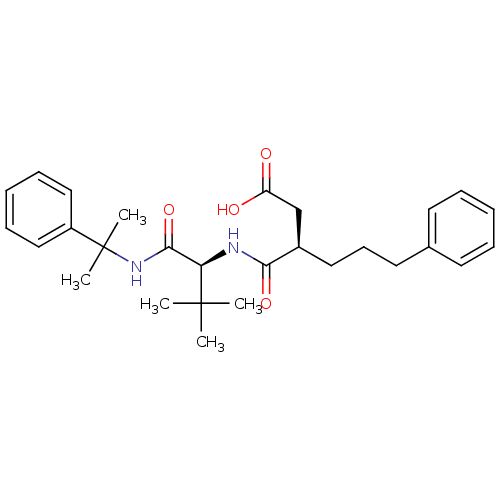 ((R)-3-(((S)-3,3-dimethyl-1-oxo-1-(2-phenylpropan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |