 Found 89 hits with Last Name = 'cale' and Initial = 'j'
Found 89 hits with Last Name = 'cale' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92479
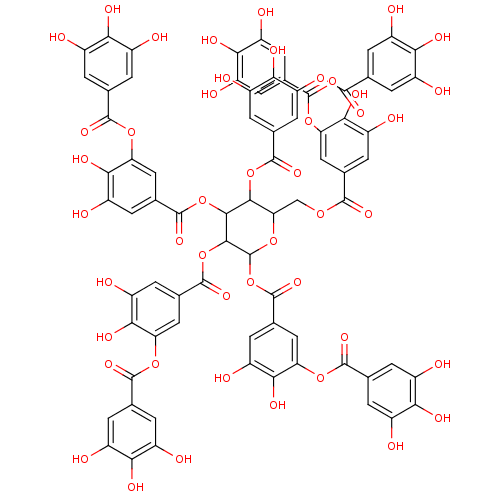
(Tannic Acid, A | Tannic acid)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OCC1OC(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92479

(Tannic Acid, A | Tannic acid)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OCC1OC(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92479

(Tannic Acid, A | Tannic acid)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OCC1OC(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92479

(Tannic Acid, A | Tannic acid)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OCC1OC(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92485
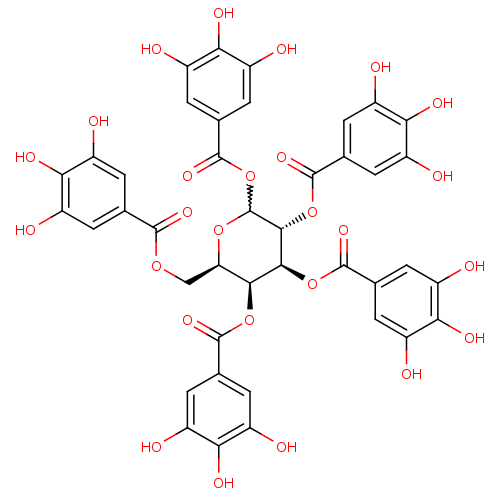
(CDE-066 | US9120744, CDE-066)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1OC(OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,w:15.16| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92485

(CDE-066 | US9120744, CDE-066)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1OC(OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,w:15.16| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175527
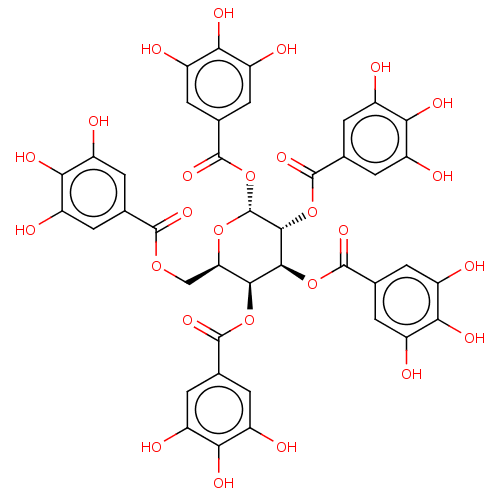
(US9120744, CDE-003)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175526
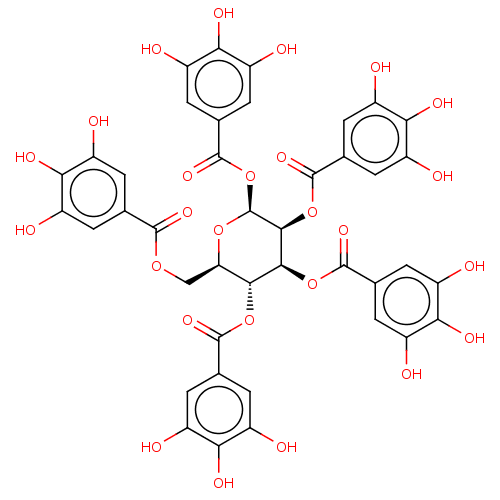
(US9120744, CDE-002)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175528
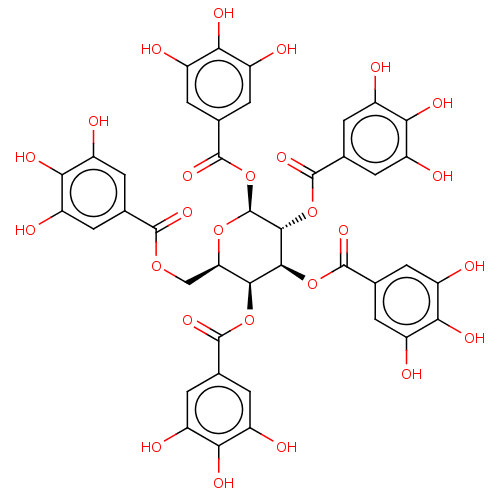
(US9120744, CDE-004)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175525
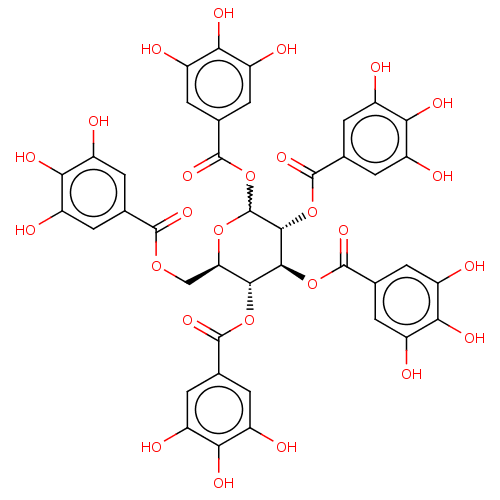
(US9120744, CDE-001 (or 073))Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1OC(OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,w:15.16| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92485

(CDE-066 | US9120744, CDE-066)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1OC(OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,w:15.16| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92485

(CDE-066 | US9120744, CDE-066)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1OC(OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,w:15.16| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33+,34+,35-,41?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92486
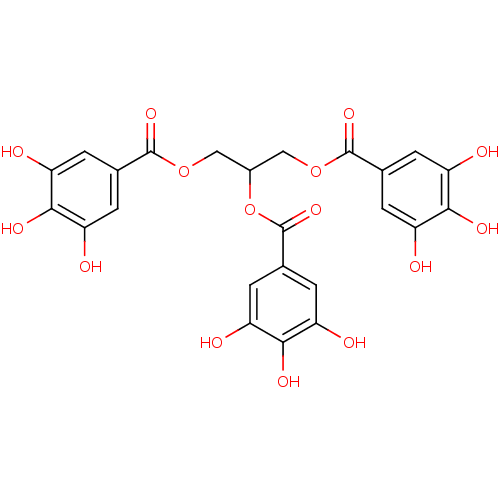
(CDE-082 | US9120744, CDE-082)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCC(COC(=O)c1cc(O)c(O)c(O)c1)OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C24H20O15/c25-13-1-9(2-14(26)19(13)31)22(34)37-7-12(39-24(36)11-5-17(29)21(33)18(30)6-11)8-38-23(35)10-3-15(27)20(32)16(28)4-10/h1-6,12,25-33H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92486

(CDE-082 | US9120744, CDE-082)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCC(COC(=O)c1cc(O)c(O)c(O)c1)OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C24H20O15/c25-13-1-9(2-14(26)19(13)31)22(34)37-7-12(39-24(36)11-5-17(29)21(33)18(30)6-11)8-38-23(35)10-3-15(27)20(32)16(28)4-10/h1-6,12,25-33H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92487
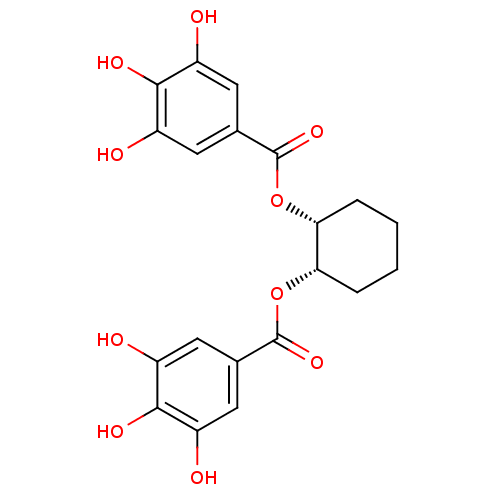
(CDE-031 | US9120744, CDE-031)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92487

(CDE-031 | US9120744, CDE-031)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92482
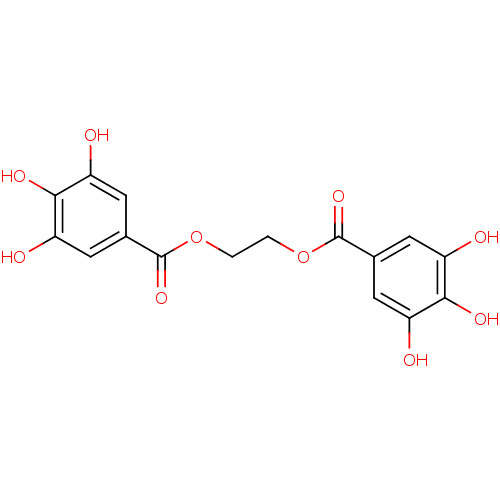
(CDE-008 | US9120744, CDE-008)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCCOC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C16H14O10/c17-9-3-7(4-10(18)13(9)21)15(23)25-1-2-26-16(24)8-5-11(19)14(22)12(20)6-8/h3-6,17-22H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175546
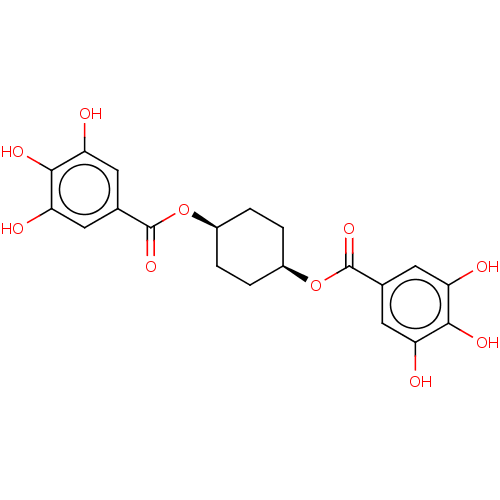
(US9120744, CDE-061)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@H]1CC[C@H](CC1)OC(=O)c1cc(O)c(O)c(O)c1 |r,wU:15.19,12.12,(6.67,-5.39,;6.67,-3.85,;5.33,-3.08,;5.33,-1.54,;6.67,-.77,;8,-1.54,;9.34,-.77,;8,-3.08,;9.34,-3.85,;4,-.77,;4,.77,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;,1.54,;1.33,.77,;-2.67,1.54,;-4,.77,;-4,-.77,;-5.33,1.54,;-6.67,.77,;-8,1.54,;-9.34,.77,;-8,3.08,;-9.34,3.85,;-6.67,3.85,;-6.67,5.39,;-5.33,3.08,)| Show InChI InChI=1S/C20H20O10/c21-13-5-9(6-14(22)17(13)25)19(27)29-11-1-2-12(4-3-11)30-20(28)10-7-15(23)18(26)16(24)8-10/h5-8,11-12,21-26H,1-4H2/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175544
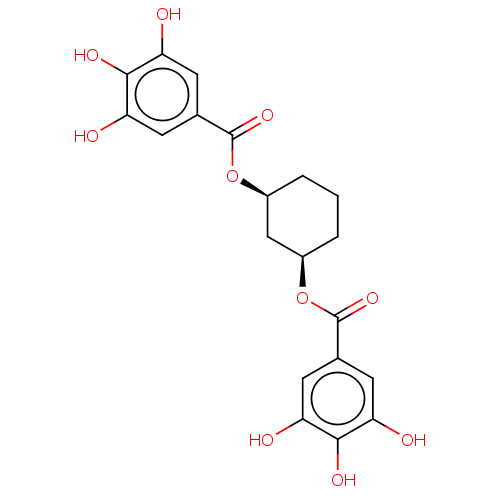
(US9120744, CDE-058)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@H]1CCC[C@H](C1)OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C20H20O10/c21-13-4-9(5-14(22)17(13)25)19(27)29-11-2-1-3-12(8-11)30-20(28)10-6-15(23)18(26)16(24)7-10/h4-7,11-12,21-26H,1-3,8H2/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175558
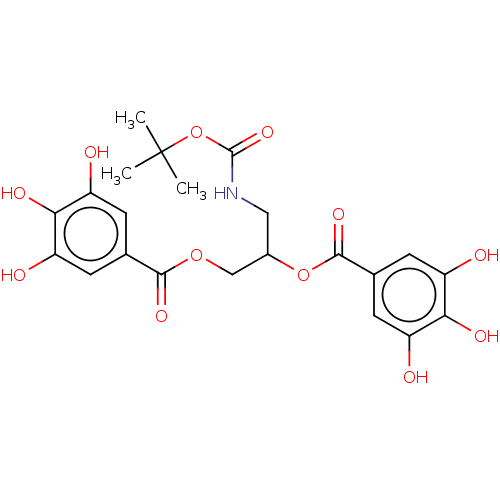
(US9120744, CDE-075)Show SMILES CC(C)(C)OC(=O)NCC(COC(=O)c1cc(O)c(O)c(O)c1)OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H25NO12/c1-22(2,3)35-21(32)23-8-12(34-20(31)11-6-15(26)18(29)16(27)7-11)9-33-19(30)10-4-13(24)17(28)14(25)5-10/h4-7,12,24-29H,8-9H2,1-3H3,(H,23,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92484
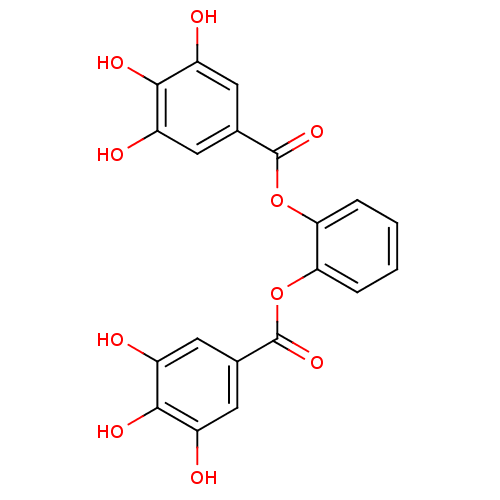
(CDE-056 | US9120744, CDE-056)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1ccccc1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H14O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h1-8,21-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92486

(CDE-082 | US9120744, CDE-082)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCC(COC(=O)c1cc(O)c(O)c(O)c1)OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C24H20O15/c25-13-1-9(2-14(26)19(13)31)22(34)37-7-12(39-24(36)11-5-17(29)21(33)18(30)6-11)8-38-23(35)10-3-15(27)20(32)16(28)4-10/h1-6,12,25-33H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92484

(CDE-056 | US9120744, CDE-056)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1ccccc1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H14O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h1-8,21-26H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175548
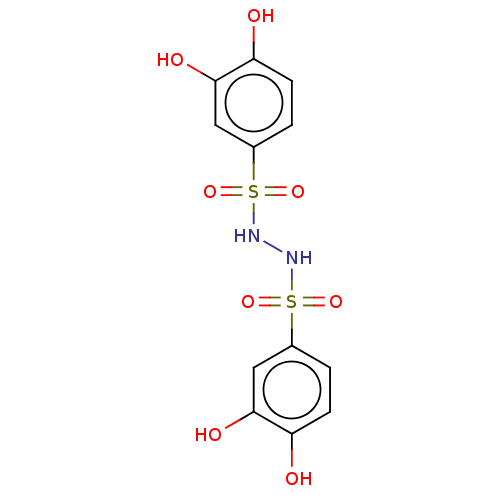
(US9120744, CDE-059)Show SMILES Oc1ccc(cc1O)S(=O)(=O)NNS(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C12H12N2O8S2/c15-9-3-1-7(5-11(9)17)23(19,20)13-14-24(21,22)8-2-4-10(16)12(18)6-8/h1-6,13-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92483

(CDE-034 | US9120744, CDE-034)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92487

(CDE-031 | US9120744, CDE-031)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92482

(CDE-008 | US9120744, CDE-008)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCCOC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C16H14O10/c17-9-3-7(4-10(18)13(9)21)15(23)25-1-2-26-16(24)8-5-11(19)14(22)12(20)6-8/h3-6,17-22H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92483

(CDE-034 | US9120744, CDE-034)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92487

(CDE-031 | US9120744, CDE-031)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92480
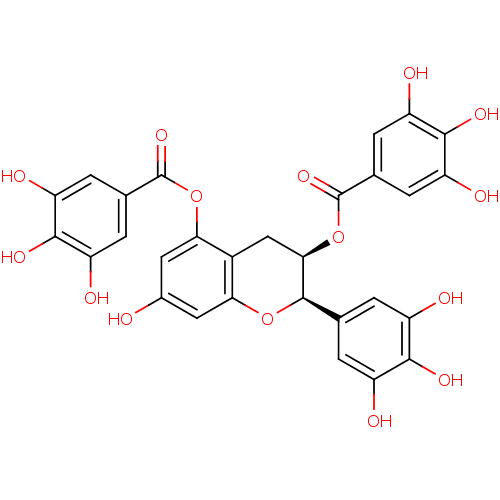
(Epigallocatechin-3,5-Digallate, C)Show SMILES Oc1cc(OC(=O)c2cc(O)c(O)c(O)c2)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C29H22O15/c30-13-7-21-14(22(8-13)43-28(40)11-3-17(33)25(38)18(34)4-11)9-23(27(42-21)10-1-15(31)24(37)16(32)2-10)44-29(41)12-5-19(35)26(39)20(36)6-12/h1-8,23,27,30-39H,9H2/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92482

(CDE-008 | US9120744, CDE-008)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCCOC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C16H14O10/c17-9-3-7(4-10(18)13(9)21)15(23)25-1-2-26-16(24)8-5-11(19)14(22)12(20)6-8/h3-6,17-22H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92483

(CDE-034 | US9120744, CDE-034)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 644 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM92484

(CDE-056 | US9120744, CDE-056)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1ccccc1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H14O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h1-8,21-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92483

(CDE-034 | US9120744, CDE-034)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175552
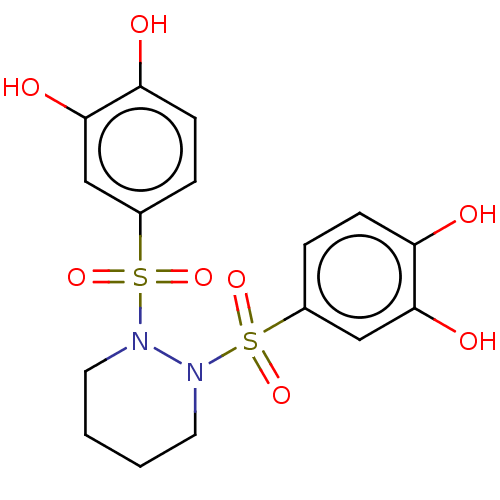
(US9120744, CDE-074)Show SMILES Oc1ccc(cc1O)S(=O)(=O)N1CCCCN1S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C16H18N2O8S2/c19-13-5-3-11(9-15(13)21)27(23,24)17-7-1-2-8-18(17)28(25,26)12-4-6-14(20)16(22)10-12/h3-6,9-10,19-22H,1-2,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM92484

(CDE-056 | US9120744, CDE-056)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1ccccc1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H14O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h1-8,21-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM31712
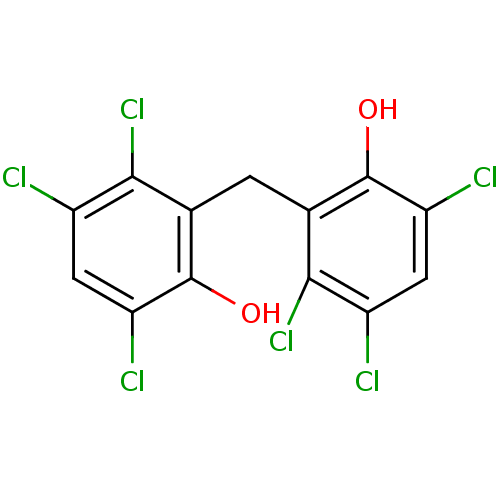
(HEXACHLOROPHENE | Hexach-lorophene | MLS000028433 ...)Show SMILES Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl Show InChI InChI=1S/C13H6Cl6O2/c14-6-2-8(16)12(20)4(10(6)18)1-5-11(19)7(15)3-9(17)13(5)21/h2-3,20-21H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50041419
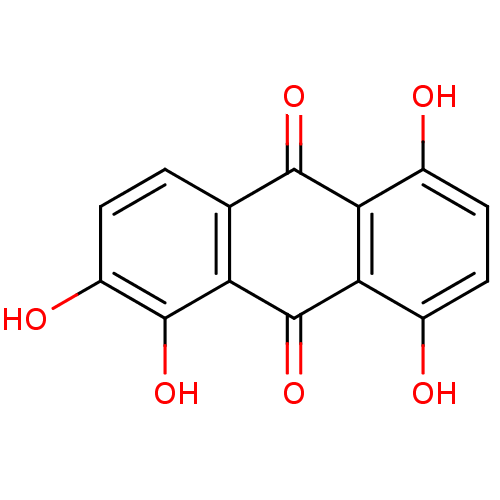
(1,2,5,8-tetrahydroxy-9,10-anthracenedione | 1,2,5,...)Show InChI InChI=1S/C14H8O6/c15-6-3-4-7(16)11-10(6)12(18)5-1-2-8(17)13(19)9(5)14(11)20/h1-4,15-17,19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50085536

(3,4,5-Trihydroxybenzoate, X | 3,4,5-trihydroxybenz...)Show InChI InChI=1S/C7H6O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,8-10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM175534
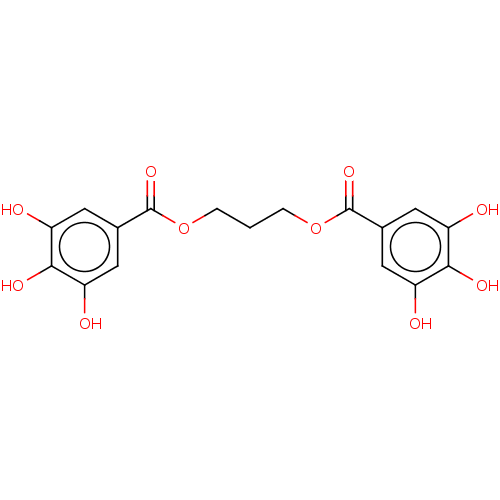
(US9120744, CDE-013)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCCCOC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C17H16O10/c18-10-4-8(5-11(19)14(10)22)16(24)26-2-1-3-27-17(25)9-6-12(20)15(23)13(21)7-9/h4-7,18-23H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human glycosylated PAI1 using urokinase type plasminogen activator substrate by direct chromogenic assay |
J Biol Chem 282: 9288-96 (2007)
Article DOI: 10.1074/jbc.M611642200
BindingDB Entry DOI: 10.7270/Q2FF3S51 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human non-glycosylated PAI1 using urokinase type plasminogen activator substrate by direct chromogenic assay |
J Biol Chem 282: 9288-96 (2007)
Article DOI: 10.1074/jbc.M611642200
BindingDB Entry DOI: 10.7270/Q2FF3S51 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92487

(CDE-031 | US9120744, CDE-031)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1CCCC[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H20O10/c21-11-5-9(6-12(22)17(11)25)19(27)29-15-3-1-2-4-16(15)30-20(28)10-7-13(23)18(26)14(24)8-10/h5-8,15-16,21-26H,1-4H2/t15-,16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92482

(CDE-008 | US9120744, CDE-008)Show SMILES Oc1cc(cc(O)c1O)C(=O)OCCOC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C16H14O10/c17-9-3-7(4-10(18)13(9)21)15(23)25-1-2-26-16(24)8-5-11(19)14(22)12(20)6-8/h3-6,17-22H,1-2H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50310301
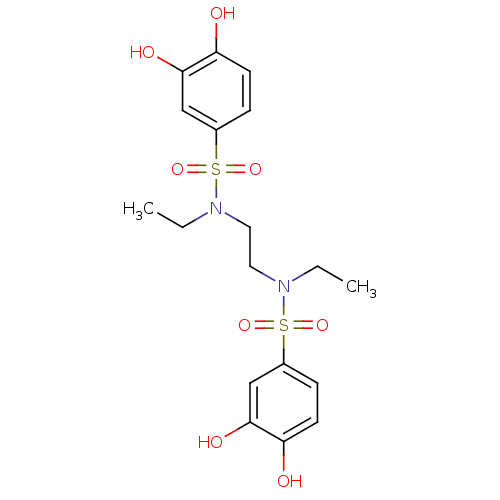
(CHEMBL599742 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...)Show SMILES CCN(CCN(CC)S(=O)(=O)c1ccc(O)c(O)c1)S(=O)(=O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H24N2O8S2/c1-3-19(29(25,26)13-5-7-15(21)17(23)11-13)9-10-20(4-2)30(27,28)14-6-8-16(22)18(24)12-14/h5-8,11-12,21-24H,3-4,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY
US Patent
| Assay Description
To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... |
US Patent US9120744 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RN8 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human non-glycosylated PAI1 using tissue type plasminogen activator substrate by direct chromogenic assay |
J Biol Chem 282: 9288-96 (2007)
Article DOI: 10.1074/jbc.M611642200
BindingDB Entry DOI: 10.7270/Q2FF3S51 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM92479

(Tannic Acid, A | Tannic acid)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OCC1OC(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C(OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)C1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan
| Assay Description
Enzyme activity assay using human and murine PAI-1. |
J Biol Chem 285: 7892-902 (2010)
Article DOI: 10.1074/jbc.M109.067967
BindingDB Entry DOI: 10.7270/Q2ZC81FC |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human glycosylated PAI1 using tissue type plasminogen activator substrate by direct chromogenic assay |
J Biol Chem 282: 9288-96 (2007)
Article DOI: 10.1074/jbc.M611642200
BindingDB Entry DOI: 10.7270/Q2FF3S51 |
More data for this
Ligand-Target Pair | |
Plasminogen activator inhibitor 1
(Homo sapiens (Human)) | BDBM50149275

(2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...)Show SMILES OC(=O)C(=O)c1cn(Cc2ccccc2)c2ccc(cc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H16F3NO4/c25-24(26,27)32-18-9-6-16(7-10-18)17-8-11-21-19(12-17)20(22(29)23(30)31)14-28(21)13-15-4-2-1-3-5-15/h1-12,14H,13H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School
Curated by ChEMBL
| Assay Description
Inhibition of vitronectin binding to human PAI1 |
J Biol Chem 282: 9288-96 (2007)
Article DOI: 10.1074/jbc.M611642200
BindingDB Entry DOI: 10.7270/Q2FF3S51 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data