 Found 104 hits with Last Name = 'canet' and Initial = 'e'
Found 104 hits with Last Name = 'canet' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50290462
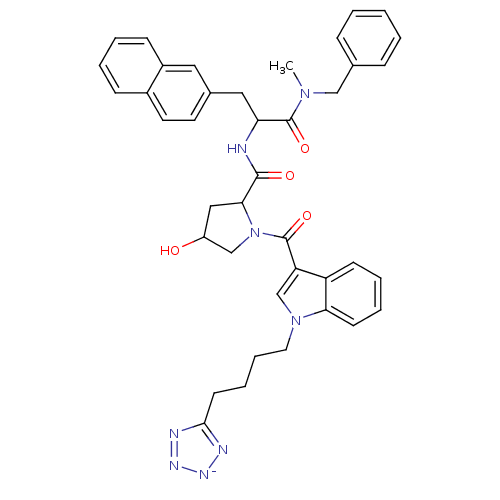
(CHEMBL305119 | potassium 2N-[1-benzyl(methyl)carba...)Show SMILES CN(Cc1ccccc1)C(=O)C(Cc1ccc2ccccc2c1)NC(=O)C1CC(O)CN1C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12 Show InChI InChI=1S/C40H42N8O4/c1-46(24-27-11-3-2-4-12-27)40(52)34(22-28-18-19-29-13-5-6-14-30(29)21-28)41-38(50)36-23-31(49)25-48(36)39(51)33-26-47(35-16-8-7-15-32(33)35)20-10-9-17-37-42-44-45-43-37/h2-8,11-16,18-19,21,26,31,34,36,49H,9-10,17,20,22-25H2,1H3,(H2,41,42,43,44,45,50)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-1 receptor was determined in a radioligand binding assay using IM9 human lymphoblastoma cell line. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Rattus norvegicus) | BDBM50290462

(CHEMBL305119 | potassium 2N-[1-benzyl(methyl)carba...)Show SMILES CN(Cc1ccccc1)C(=O)C(Cc1ccc2ccccc2c1)NC(=O)C1CC(O)CN1C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12 Show InChI InChI=1S/C40H42N8O4/c1-46(24-27-11-3-2-4-12-27)40(52)34(22-28-18-19-29-13-5-6-14-30(29)21-28)41-38(50)36-23-31(49)25-48(36)39(51)33-26-47(35-16-8-7-15-32(33)35)20-10-9-17-37-42-44-45-43-37/h2-8,11-16,18-19,21,26,31,34,36,49H,9-10,17,20,22-25H2,1H3,(H2,41,42,43,44,45,50)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-3 receptor was determined in vitro using isolated rat portal vein. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Oryctolagus cuniculus) | BDBM50290461
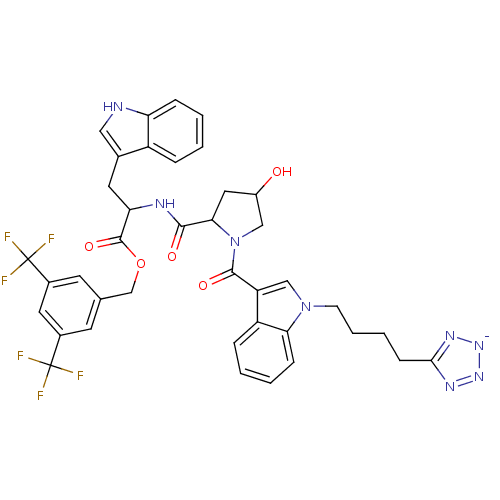
(CHEMBL65468 | potassium 3,5-di(trifluoromethyl)ben...)Show SMILES OC1CC(N(C1)C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C39H36F6N8O5/c40-38(41,42)24-13-22(14-25(16-24)39(43,44)45)21-58-37(57)31(15-23-18-46-30-9-3-1-7-27(23)30)47-35(55)33-17-26(54)19-53(33)36(56)29-20-52(32-10-4-2-8-28(29)32)12-6-5-11-34-48-50-51-49-34/h1-4,7-10,13-14,16,18,20,26,31,33,46,54H,5-6,11-12,15,17,19,21H2,(H2,47,48,49,50,51,55)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-2 receptor was determined in vitro using isolated rabbit pulmonary artery. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Rattus norvegicus) | BDBM50290461

(CHEMBL65468 | potassium 3,5-di(trifluoromethyl)ben...)Show SMILES OC1CC(N(C1)C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C39H36F6N8O5/c40-38(41,42)24-13-22(14-25(16-24)39(43,44)45)21-58-37(57)31(15-23-18-46-30-9-3-1-7-27(23)30)47-35(55)33-17-26(54)19-53(33)36(56)29-20-52(32-10-4-2-8-28(29)32)12-6-5-11-34-48-50-51-49-34/h1-4,7-10,13-14,16,18,20,26,31,33,46,54H,5-6,11-12,15,17,19,21H2,(H2,47,48,49,50,51,55)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-3 receptor was determined in vitro using isolated rat portal vein. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Oryctolagus cuniculus) | BDBM50290462

(CHEMBL305119 | potassium 2N-[1-benzyl(methyl)carba...)Show SMILES CN(Cc1ccccc1)C(=O)C(Cc1ccc2ccccc2c1)NC(=O)C1CC(O)CN1C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12 Show InChI InChI=1S/C40H42N8O4/c1-46(24-27-11-3-2-4-12-27)40(52)34(22-28-18-19-29-13-5-6-14-30(29)21-28)41-38(50)36-23-31(49)25-48(36)39(51)33-26-47(35-16-8-7-15-32(33)35)20-10-9-17-37-42-44-45-43-37/h2-8,11-16,18-19,21,26,31,34,36,49H,9-10,17,20,22-25H2,1H3,(H2,41,42,43,44,45,50)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-2 receptor was determined in vitro using isolated rabbit pulmonary artery. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50290461

(CHEMBL65468 | potassium 3,5-di(trifluoromethyl)ben...)Show SMILES OC1CC(N(C1)C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C39H36F6N8O5/c40-38(41,42)24-13-22(14-25(16-24)39(43,44)45)21-58-37(57)31(15-23-18-46-30-9-3-1-7-27(23)30)47-35(55)33-17-26(54)19-53(33)36(56)29-20-52(32-10-4-2-8-28(29)32)12-6-5-11-34-48-50-51-49-34/h1-4,7-10,13-14,16,18,20,26,31,33,46,54H,5-6,11-12,15,17,19,21H2,(H2,47,48,49,50,51,55)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-1 receptor was determined in vitro using isolated rabbit vena cava. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50290461

(CHEMBL65468 | potassium 3,5-di(trifluoromethyl)ben...)Show SMILES OC1CC(N(C1)C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C39H36F6N8O5/c40-38(41,42)24-13-22(14-25(16-24)39(43,44)45)21-58-37(57)31(15-23-18-46-30-9-3-1-7-27(23)30)47-35(55)33-17-26(54)19-53(33)36(56)29-20-52(32-10-4-2-8-28(29)32)12-6-5-11-34-48-50-51-49-34/h1-4,7-10,13-14,16,18,20,26,31,33,46,54H,5-6,11-12,15,17,19,21H2,(H2,47,48,49,50,51,55)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic potency for NK-1 receptor was determined in a radioligand binding assay, using IM9 human lymphoblastoma cell line. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50290462

(CHEMBL305119 | potassium 2N-[1-benzyl(methyl)carba...)Show SMILES CN(Cc1ccccc1)C(=O)C(Cc1ccc2ccccc2c1)NC(=O)C1CC(O)CN1C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12 Show InChI InChI=1S/C40H42N8O4/c1-46(24-27-11-3-2-4-12-27)40(52)34(22-28-18-19-29-13-5-6-14-30(29)21-28)41-38(50)36-23-31(49)25-48(36)39(51)33-26-47(35-16-8-7-15-32(33)35)20-10-9-17-37-42-44-45-43-37/h2-8,11-16,18-19,21,26,31,34,36,49H,9-10,17,20,22-25H2,1H3,(H2,41,42,43,44,45,50)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic potency for NK-2 receptor was determined in vitro, using isolated rabbit pulmonary artery. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50290462

(CHEMBL305119 | potassium 2N-[1-benzyl(methyl)carba...)Show SMILES CN(Cc1ccccc1)C(=O)C(Cc1ccc2ccccc2c1)NC(=O)C1CC(O)CN1C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12 Show InChI InChI=1S/C40H42N8O4/c1-46(24-27-11-3-2-4-12-27)40(52)34(22-28-18-19-29-13-5-6-14-30(29)21-28)41-38(50)36-23-31(49)25-48(36)39(51)33-26-47(35-16-8-7-15-32(33)35)20-10-9-17-37-42-44-45-43-37/h2-8,11-16,18-19,21,26,31,34,36,49H,9-10,17,20,22-25H2,1H3,(H2,41,42,43,44,45,50)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic potency for NK-2 receptor was determined in vitro, using isolated rabbit pulmonary artery. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50290461

(CHEMBL65468 | potassium 3,5-di(trifluoromethyl)ben...)Show SMILES OC1CC(N(C1)C(=O)c1cn(CCCCc2nn[n-]n2)c2ccccc12)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C39H36F6N8O5/c40-38(41,42)24-13-22(14-25(16-24)39(43,44)45)21-58-37(57)31(15-23-18-46-30-9-3-1-7-27(23)30)47-35(55)33-17-26(54)19-53(33)36(56)29-20-52(32-10-4-2-8-28(29)32)12-6-5-11-34-48-50-51-49-34/h1-4,7-10,13-14,16,18,20,26,31,33,46,54H,5-6,11-12,15,17,19,21H2,(H2,47,48,49,50,51,55)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for NK-2 receptor was determined in vitro using isolated rabbit pulmonary artery. |
Bioorg Med Chem Lett 7: 203-208 (1997)
Article DOI: 10.1016/S0960-894X(96)00604-X
BindingDB Entry DOI: 10.7270/Q2VD6ZGN |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058495
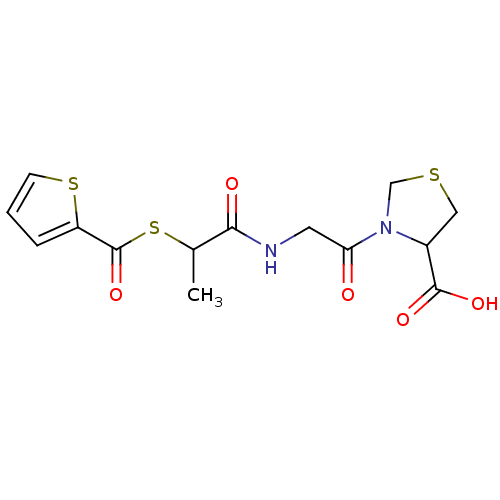
(3-{2-[2-(Thiophene-2-carbonylsulfanyl)-propionylam...)Show InChI InChI=1S/C14H16N2O5S3/c1-8(24-14(21)10-3-2-4-23-10)12(18)15-5-11(17)16-7-22-6-9(16)13(19)20/h2-4,8-9H,5-7H2,1H3,(H,15,18)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058491

(3-{4-[2-(4-{1-[4-(2-Carboxy-2-methyl-propane-1-sul...)Show SMILES CC(C)(CS(=O)(=O)c1ccc(OC(=O)C(C)(C)c2ccc(cc2)C(C)(C)C(=O)Oc2ccc(cc2)S(=O)(=O)CC(C)(C)C(O)=O)cc1)C(O)=O Show InChI InChI=1S/C36H42O12S2/c1-33(2,29(37)38)21-49(43,44)27-17-13-25(14-18-27)47-31(41)35(5,6)23-9-11-24(12-10-23)36(7,8)32(42)48-26-15-19-28(20-16-26)50(45,46)22-34(3,4)30(39)40/h9-20H,21-22H2,1-8H3,(H,37,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045033
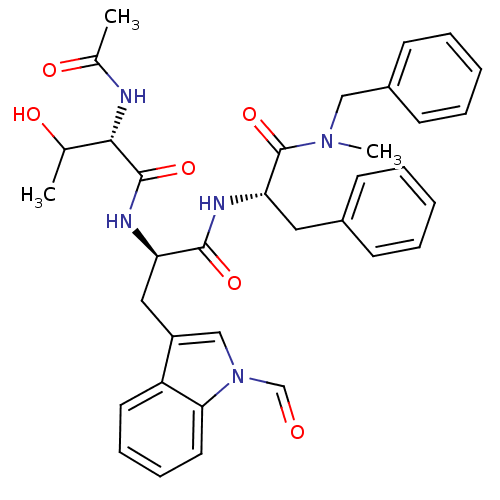
(1N-[1-[1-benzyl(methyl)carbamoyl-2-phenyl-(1S)-eth...)Show SMILES CC(O)[C@H](NC(C)=O)C(=O)N[C@H](Cc1cn(C=O)c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C35H39N5O6/c1-23(42)32(36-24(2)43)34(45)37-29(19-27-21-40(22-41)31-17-11-10-16-28(27)31)33(44)38-30(18-25-12-6-4-7-13-25)35(46)39(3)20-26-14-8-5-9-15-26/h4-17,21-23,29-30,32,42H,18-20H2,1-3H3,(H,36,43)(H,37,45)(H,38,44)/t23?,29-,30+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-1 receptor transfected on CHO cells using [125I]-Tyr] SP as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058493

((R)-2-{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:35.39,(13.8,-10.72,;12.68,-10.08,;11.36,-10.85,;12.7,-8.54,;11.37,-7.77,;10.02,-8.54,;10.02,-10.08,;8.71,-7.77,;7.36,-8.53,;6.03,-7.76,;6.04,-6.22,;7.38,-5.45,;8.71,-6.22,;4.73,-5.43,;4.73,-3.89,;3.38,-6.2,;2.06,-5.43,;1.28,-6.77,;2.82,-4.11,;.72,-4.66,;-.61,-5.43,;-1.95,-4.66,;-1.95,-3.12,;-3.27,-2.35,;-.61,-2.35,;.72,-3.12,;14.13,-7.67,;14.29,-6.13,;15.37,-8.57,;15.35,-10.11,;16.95,-10.2,;16.57,-8.71,;18.11,-8.71,;18.09,-10.25,;16.76,-11,;16.78,-7.94,;16.94,-6.42,;15.7,-5.5,;18.75,-6.17,;18.88,-4.44,;17.74,-3.41,;16.28,-3.89,;18.07,-1.9,;20.35,-3.98,;20.67,-2.47,;21.5,-5,;22.53,-5.95,;21.41,-6.61,;23.08,-4.76,)| Show InChI InChI=1S/C33H38ClF3N4O7S/c1-17(2)25(28(42)33(35,36)37)38-31(45)27-19-9-13-23(14-10-19)41(27)32(46)26(18(3)4)39-29(43)20-5-7-21(8-6-20)30(44)40-49(47,48)24-15-11-22(34)12-16-24/h5-8,11-12,15-19,23,25-27H,9-10,13-14H2,1-4H3,(H,38,45)(H,39,43)(H,40,44)/t19?,23?,25?,26?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045040
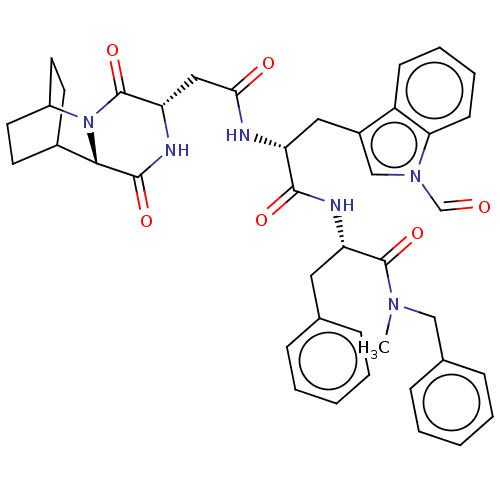
(CHEMBL3144343 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:43.46,11.11,39.42,22.24,THB:41:43:46.45:48.49,(10.33,-13,;9.17,-14.02,;9.47,-15.53,;10.93,-16.02,;11.23,-17.53,;12.69,-18.03,;13.85,-17.01,;13.55,-15.5,;12.09,-15.01,;7.71,-13.52,;6.56,-14.54,;7.41,-12.01,;8.57,-11,;8.26,-9.49,;9.42,-8.47,;9.12,-6.96,;7.66,-6.47,;6.5,-7.48,;6.81,-8.99,;5.95,-11.52,;5.65,-10.01,;5.99,-8.51,;4.19,-9.52,;3.03,-10.53,;3.34,-12.04,;4.73,-12.69,;4.56,-14.22,;5.69,-15.26,;5.35,-16.76,;3.05,-14.52,;2.26,-15.84,;.72,-15.82,;-.03,-14.48,;.75,-13.16,;2.29,-13.18,;3.89,-8.01,;2.43,-7.51,;1.27,-8.53,;2.13,-6,;.67,-5.51,;.37,-4,;-1.09,-3.51,;-1.39,-2,;-2.25,-4.52,;-3.61,-3.99,;-5.11,-4.65,;-4.91,-6.06,;-3.34,-5.39,;-3.08,-3.45,;-3.54,-2.32,;-1.95,-6.03,;-.49,-6.53,;-.19,-8.04,)| Show InChI InChI=1S/C41H44N6O6/c1-45(23-27-12-6-3-7-13-27)40(52)33(20-26-10-4-2-5-11-26)43-38(50)32(21-29-24-46(25-48)35-15-9-8-14-31(29)35)42-36(49)22-34-41(53)47-30-18-16-28(17-19-30)37(47)39(51)44-34/h2-15,24-25,28,30,32-34,37H,16-23H2,1H3,(H,42,49)(H,43,50)(H,44,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058492

((R)-1-{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1C2CCCCC2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C34H40ClF3N4O7S/c1-18(2)27(29(43)34(36,37)38)39-32(46)26-17-22-7-5-6-8-25(22)42(26)33(47)28(19(3)4)40-30(44)20-9-11-21(12-10-20)31(45)41-50(48,49)24-15-13-23(35)14-16-24/h9-16,18-19,22,25-28H,5-8,17H2,1-4H3,(H,39,46)(H,40,44)(H,41,45)/t22?,25?,26-,27?,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058474
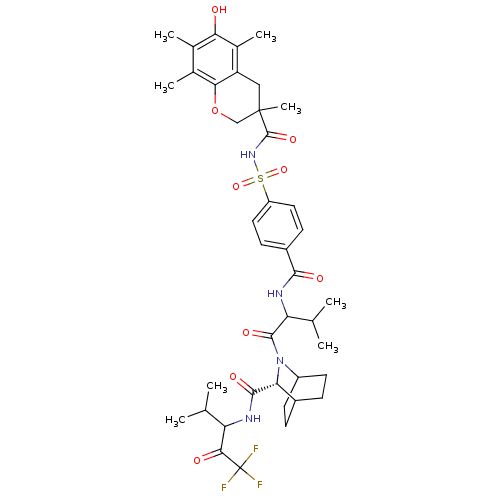
((R)-2-(2-{4-[(6-Hydroxy-3,5,7,8-tetramethyl-chroma...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)C1(C)COc2c(C)c(C)c(O)c(C)c2C1)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:43.48,(14.83,-15.75,;13.74,-15.12,;12.39,-15.89,;13.74,-13.58,;12.42,-12.81,;11.08,-13.58,;11.08,-15.12,;9.75,-12.81,;9.75,-11.24,;8.42,-10.49,;7.1,-11.24,;7.09,-12.8,;8.42,-13.57,;5.77,-10.47,;7.04,-9.44,;5.13,-11.75,;4.5,-9.54,;3.89,-8.13,;4.95,-7.03,;2.61,-7.26,;1.74,-8.54,;3.35,-5.91,;2.55,-4.6,;1.03,-4.65,;.24,-3.35,;.99,-1.97,;-1.3,-3.37,;-2.07,-2.03,;-2.04,-4.73,;-3.58,-4.79,;-1.24,-6.03,;-1.98,-7.39,;.3,-6,;1.09,-7.29,;15.17,-12.7,;15.35,-11.16,;16.4,-13.6,;16.4,-15.14,;18.01,-15.24,;17.61,-13.74,;19.15,-13.74,;19.13,-15.28,;17.8,-16.04,;17.82,-12.97,;17.98,-11.45,;16.75,-10.53,;19.79,-11.21,;19.93,-9.47,;18.78,-8.45,;19.12,-6.94,;17.32,-8.93,;21.4,-9.01,;21.72,-7.51,;22.53,-10.04,;23.59,-10.98,;22.46,-11.65,;24.14,-9.8,)| Show InChI InChI=1S/C40H51F3N4O9S/c1-19(2)29(34(49)40(41,42)43)44-36(51)31-24-9-13-26(14-10-24)47(31)37(52)30(20(3)4)45-35(50)25-11-15-27(16-12-25)57(54,55)46-38(53)39(8)17-28-23(7)32(48)21(5)22(6)33(28)56-18-39/h11-12,15-16,19-20,24,26,29-31,48H,9-10,13-14,17-18H2,1-8H3,(H,44,51)(H,45,50)(H,46,53)/t24?,26?,29?,30?,31-,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045036
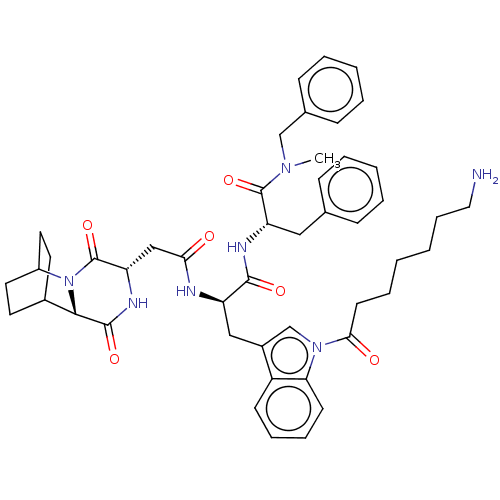
(7-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:50.53,46.49,22.24,wD:11.11,THB:48:50:53.52:55.56,(11.12,-10.02,;9.98,-8.97,;11.14,-7.96,;12.6,-8.45,;13.76,-7.43,;15.21,-7.93,;15.52,-9.44,;14.36,-10.45,;12.9,-9.96,;8.52,-8.48,;8.22,-6.97,;7.37,-9.5,;7.67,-11.01,;9.13,-11.5,;10.29,-10.48,;11.74,-10.98,;12.05,-12.49,;10.89,-13.5,;9.43,-13.01,;5.91,-9,;4.75,-10.02,;5.05,-11.53,;3.29,-9.52,;2.14,-10.54,;2.44,-12.05,;3.84,-12.69,;3.66,-14.22,;4.79,-15.27,;6.26,-14.81,;4.45,-16.77,;5.58,-17.81,;5.25,-19.32,;6.38,-20.36,;6.04,-21.86,;7.17,-22.91,;6.83,-24.41,;2.15,-14.53,;1.36,-15.85,;-.18,-15.83,;-.93,-14.49,;-.15,-13.16,;1.39,-13.18,;2.99,-8.01,;4.15,-7,;5.61,-7.49,;3.85,-5.49,;2.39,-4.99,;2.09,-3.48,;.63,-2.99,;.32,-1.48,;-.53,-4.01,;-1.89,-3.47,;-3.39,-4.13,;-3.19,-5.54,;-1.63,-4.87,;-1.36,-2.93,;-1.82,-1.8,;-.23,-5.52,;1.23,-6.01,;1.53,-7.52,)| Show InChI InChI=1S/C47H57N7O6/c1-52(29-32-16-8-5-9-17-32)46(59)38(26-31-14-6-4-7-15-31)50-44(57)37(27-34-30-53(40-19-12-11-18-36(34)40)42(56)20-10-2-3-13-25-48)49-41(55)28-39-47(60)54-35-23-21-33(22-24-35)43(54)45(58)51-39/h4-9,11-12,14-19,30,33,35,37-39,43H,2-3,10,13,20-29,48H2,1H3,(H,49,55)(H,50,57)(H,51,58)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-1 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058486
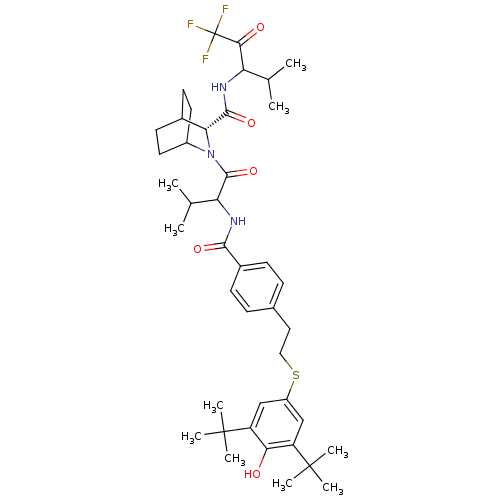
((R)-2-(2-{4-[2-(3,5-Di-tert-butyl-4-hydroxy-phenyl...)Show SMILES CC(C)C(NC(=O)c1ccc(CCSc2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)cc1)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:40.44,(14.77,-11.77,;16.11,-11,;17.21,-11.62,;16.12,-9.46,;14.79,-8.69,;13.44,-9.46,;13.44,-11,;12.13,-8.69,;10.78,-9.44,;9.44,-8.67,;9.46,-7.13,;8.13,-6.36,;8.13,-4.82,;6.8,-4.04,;6.01,-2.71,;4.49,-2.71,;3.73,-1.38,;4.5,-.06,;3.73,1.28,;6.03,-.04,;6.8,-1.39,;6.81,1.27,;6.04,2.62,;7.9,.18,;8.35,1.27,;2.18,-1.39,;1.42,-.06,;1.42,-2.73,;2.58,.1,;10.79,-6.36,;12.13,-7.13,;17.55,-8.58,;17.71,-7.04,;18.78,-9.48,;18.77,-11.02,;20.37,-11.11,;19.99,-9.63,;21.53,-9.63,;21.51,-11.16,;20.18,-11.91,;20.19,-8.86,;20.35,-7.33,;19.12,-6.42,;22.17,-7.09,;22.3,-5.36,;21.15,-4.33,;21.5,-2.82,;19.7,-4.81,;23.77,-4.89,;24.09,-3.38,;24.91,-5.91,;26.5,-5.68,;25.96,-6.87,;24.83,-7.52,)| Show InChI InChI=1S/C42H58F3N3O5S/c1-23(2)32(36(50)42(43,44)45)46-38(52)34-26-15-17-28(18-16-26)48(34)39(53)33(24(3)4)47-37(51)27-13-11-25(12-14-27)19-20-54-29-21-30(40(5,6)7)35(49)31(22-29)41(8,9)10/h11-14,21-24,26,28,32-34,49H,15-20H2,1-10H3,(H,46,52)(H,47,51)/t26?,28?,32?,33?,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058480
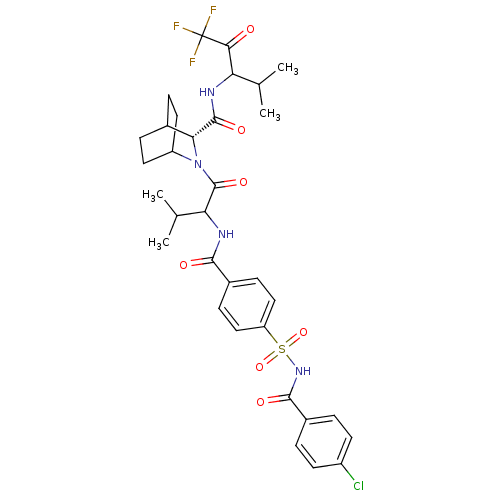
((R)-2-{2-[4-(4-Chloro-benzoylsulfamoyl)-benzoylami...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1ccc(Cl)cc1)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:35.39,(13.8,-10.72,;12.68,-10.08,;11.36,-10.85,;12.7,-8.54,;11.37,-7.77,;10.02,-8.54,;10.02,-10.08,;8.71,-7.77,;8.71,-6.22,;7.38,-5.45,;6.04,-6.22,;6.03,-7.76,;7.36,-8.53,;4.73,-5.43,;4.09,-6.72,;6,-4.41,;3.44,-4.5,;2.83,-3.09,;3.89,-1.99,;1.39,-2.55,;.2,-3.51,;-1.22,-2.96,;-1.46,-1.45,;-2.91,-.9,;-.28,-.48,;1.17,-1.04,;14.13,-7.67,;14.29,-6.13,;15.37,-8.57,;15.35,-10.11,;16.95,-10.2,;16.57,-8.71,;18.11,-8.71,;18.09,-10.25,;16.76,-11,;16.78,-7.94,;16.94,-6.42,;15.7,-5.5,;18.75,-6.17,;18.88,-4.44,;17.74,-3.41,;16.28,-3.89,;18.07,-1.9,;20.35,-3.98,;20.67,-2.47,;21.5,-5,;22.53,-5.95,;21.41,-6.61,;23.08,-4.76,)| Show InChI InChI=1S/C33H38ClF3N4O7S/c1-17(2)25(28(42)33(35,36)37)38-31(45)27-19-7-13-23(14-8-19)41(27)32(46)26(18(3)4)39-29(43)20-9-15-24(16-10-20)49(47,48)40-30(44)21-5-11-22(34)12-6-21/h5-6,9-12,15-19,23,25-27H,7-8,13-14H2,1-4H3,(H,38,45)(H,39,43)(H,40,44)/t19?,23?,25?,26?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058473

((2R,3aR,7aR)-1-[2-(4-{4-Chloro-3-[2-(3,5-di-tert-b...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1ccc(Cl)c(c1)S(=O)(=O)NC(=O)CSc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C50H63ClF3N5O11S3/c1-26(2)40(43(62)50(52,53)54)55-46(65)37-21-29-13-11-12-14-36(29)59(37)47(66)41(27(3)4)56-44(63)28-15-18-32(19-16-28)72(67,68)58-45(64)30-17-20-35(51)38(22-30)73(69,70)57-39(60)25-71-31-23-33(48(5,6)7)42(61)34(24-31)49(8,9)10/h15-20,22-24,26-27,29,36-37,40-41,61H,11-14,21,25H2,1-10H3,(H,55,65)(H,56,63)(H,57,60)(H,58,64)/t29-,36-,37-,40?,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058475

((R)-2-(2-{4-[2-(3,5-Di-tert-butyl-4-hydroxy-phenyl...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:44.48,(14.83,-15.75,;13.74,-15.12,;12.39,-15.89,;13.74,-13.58,;12.42,-12.81,;11.08,-13.58,;11.08,-15.12,;9.75,-12.81,;8.42,-13.57,;7.09,-12.8,;7.1,-11.24,;8.42,-10.49,;9.75,-11.24,;5.77,-10.47,;5.13,-11.75,;7.04,-9.44,;4.5,-9.54,;3.89,-8.13,;4.95,-7.03,;2.37,-7.87,;1.84,-6.42,;.33,-6.17,;-.21,-4.73,;.77,-3.54,;.22,-2.1,;2.29,-3.8,;2.82,-5.24,;3.25,-2.61,;2.73,-1.17,;4.15,-3.86,;4.78,-2.86,;-1.73,-4.49,;-2.28,-3.06,;-2.72,-5.69,;-1.11,-3.09,;15.18,-12.7,;15.35,-11.16,;16.41,-13.6,;16.41,-15.14,;17.81,-16.04,;19.13,-15.28,;19.15,-13.74,;17.61,-13.74,;18.01,-15.24,;17.82,-12.97,;17.98,-11.45,;16.75,-10.53,;19.8,-11.21,;19.93,-9.48,;18.78,-8.45,;19.12,-6.94,;17.32,-8.93,;21.4,-9.02,;21.72,-7.51,;22.53,-10.04,;22.46,-11.65,;24.14,-9.8,;23.59,-10.98,)| Show InChI InChI=1S/C42H57F3N4O8S/c1-22(2)32(36(52)42(43,44)45)46-38(54)34-25-11-15-27(16-12-25)49(34)39(55)33(23(3)4)47-37(53)26-13-17-28(18-14-26)58(56,57)48-31(50)21-24-19-29(40(5,6)7)35(51)30(20-24)41(8,9)10/h13-14,17-20,22-23,25,27,32-34,51H,11-12,15-16,21H2,1-10H3,(H,46,54)(H,47,53)(H,48,50)/t25?,27?,32?,33?,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058485

(CHEMBL2373010 | {2-Methyl-1-[(R)-3-(3,3,3-trifluor...)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F |wU:21.23,25.27,wD:3.2,(4.97,-21.94,;3.86,-21.31,;2.53,-22.08,;3.88,-19.77,;2.55,-19,;1.2,-19.77,;1.2,-21.3,;-.15,-18.99,;-1.5,-19.76,;-2.82,-18.99,;-1.5,-21.3,;-2.81,-20.54,;5.3,-18.9,;5.46,-17.36,;6.54,-19.79,;6.52,-21.33,;8.13,-21.43,;7.74,-19.93,;9.28,-19.93,;9.27,-21.47,;7.94,-22.23,;7.96,-19.16,;8.12,-17.65,;6.88,-16.73,;9.92,-17.4,;10.05,-15.67,;8.92,-14.64,;9.25,-13.13,;7.45,-15.12,;11.53,-15.21,;11.85,-13.7,;12.67,-16.23,;13.71,-17.18,;12.59,-17.84,;14.26,-15.99,)| Show InChI InChI=1S/C24H38F3N3O5/c1-12(2)16(19(31)24(25,26)27)28-20(32)18-14-8-10-15(11-9-14)30(18)21(33)17(13(3)4)29-22(34)35-23(5,6)7/h12-18H,8-11H2,1-7H3,(H,28,32)(H,29,34)/t14?,15?,16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031201

((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045039
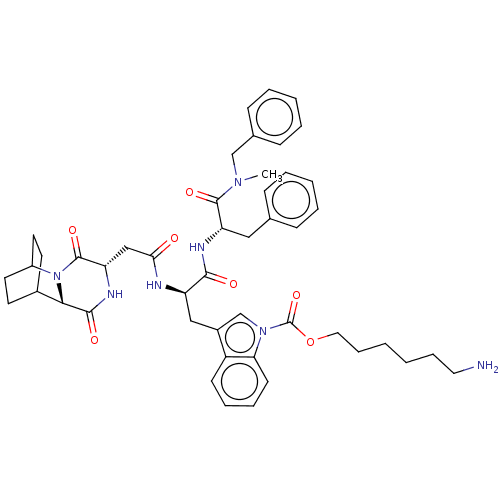
(6-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)OCCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:51.54,47.50,22.24,wD:11.12,THB:49:51:54.53:56.57,(11.28,-9.71,;10.14,-8.66,;11.3,-7.64,;12.76,-8.14,;13.92,-7.12,;15.38,-7.61,;15.68,-9.13,;14.52,-10.14,;13.06,-9.65,;8.69,-8.17,;8.38,-6.66,;7.53,-9.18,;7.83,-10.69,;9.29,-11.19,;10.45,-10.17,;11.91,-10.66,;12.21,-12.17,;11.05,-13.19,;9.59,-12.7,;6.07,-8.69,;4.91,-9.71,;5.22,-11.22,;3.46,-9.21,;2.3,-10.23,;2.6,-11.74,;4,-12.38,;3.82,-13.91,;4.95,-14.96,;6.42,-14.5,;4.61,-16.46,;5.75,-17.5,;5.41,-19.01,;6.54,-20.05,;6.2,-21.55,;7.33,-22.6,;6.99,-24.1,;8.13,-25.14,;2.31,-14.22,;1.52,-15.54,;-.02,-15.52,;-.77,-14.18,;.02,-12.85,;1.56,-12.87,;3.15,-7.7,;4.31,-6.69,;5.77,-7.18,;4.01,-5.18,;2.55,-4.68,;2.25,-3.17,;.79,-2.68,;.49,-1.17,;-.37,-3.7,;-1.73,-3.16,;-3.23,-3.82,;-3.03,-5.23,;-1.46,-4.56,;-1.2,-2.62,;-1.66,-1.49,;-.07,-5.21,;1.39,-5.7,;1.69,-7.21,)| Show InChI InChI=1S/C47H57N7O7/c1-52(29-32-16-8-5-9-17-32)45(58)38(26-31-14-6-4-7-15-31)50-43(56)37(49-41(55)28-39-46(59)54-35-22-20-33(21-23-35)42(54)44(57)51-39)27-34-30-53(40-19-11-10-18-36(34)40)47(60)61-25-13-3-2-12-24-48/h4-11,14-19,30,33,35,37-39,42H,2-3,12-13,20-29,48H2,1H3,(H,49,55)(H,50,56)(H,51,57)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058489

((2R,3aR,7aR)-1-[2-(4-{4-Chloro-3-[2-(3,5-di-tert-b...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1ccc(Cl)c(c1)S(=O)(=O)NC(=O)Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C50H63ClF3N5O11S2/c1-26(2)40(43(62)50(52,53)54)55-46(65)37-24-30-13-11-12-14-36(30)59(37)47(66)41(27(3)4)56-44(63)29-15-18-32(19-16-29)71(67,68)58-45(64)31-17-20-35(51)38(25-31)72(69,70)57-39(60)23-28-21-33(48(5,6)7)42(61)34(22-28)49(8,9)10/h15-22,25-27,30,36-37,40-41,61H,11-14,23-24H2,1-10H3,(H,55,65)(H,56,63)(H,57,60)(H,58,64)/t30-,36-,37-,40?,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058487
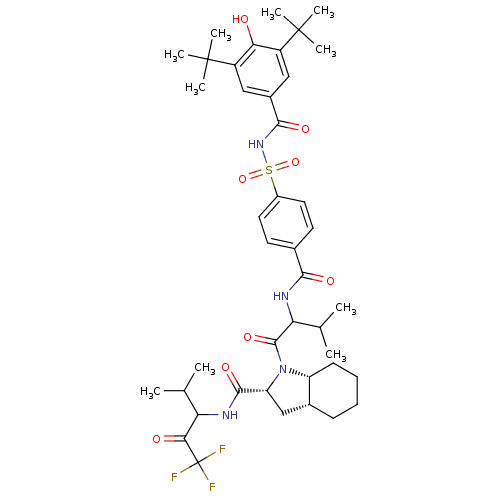
((2R,3aR,7aR)-1-{2-[4-(3,5-Di-tert-butyl-4-hydroxy-...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C42H57F3N4O8S/c1-22(2)32(35(51)42(43,44)45)46-38(54)31-21-25-13-11-12-14-30(25)49(31)39(55)33(23(3)4)47-36(52)24-15-17-27(18-16-24)58(56,57)48-37(53)26-19-28(40(5,6)7)34(50)29(20-26)41(8,9)10/h15-20,22-23,25,30-33,50H,11-14,21H2,1-10H3,(H,46,54)(H,47,52)(H,48,53)/t25-,30-,31-,32?,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058481

((2R,3aR,7aR)-1-(2-{4-[(E)-3-(3,5-Di-tert-butyl-4-e...)Show SMILES CCOCOc1c(cc(\C=C\C(=O)NS(=O)(=O)c2ccc(cc2)C(=O)NC(C(C)C)C(=O)N2[C@@H]3CCCC[C@@H]3C[C@@H]2C(=O)NC(C(C)C)C(=O)C(F)(F)F)cc1C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C47H65F3N4O9S/c1-12-62-26-63-40-33(45(6,7)8)23-29(24-34(40)46(9,10)11)17-22-37(55)53-64(60,61)32-20-18-30(19-21-32)42(57)52-39(28(4)5)44(59)54-35-16-14-13-15-31(35)25-36(54)43(58)51-38(27(2)3)41(56)47(48,49)50/h17-24,27-28,31,35-36,38-39H,12-16,25-26H2,1-11H3,(H,51,58)(H,52,57)(H,53,55)/b22-17+/t31-,35-,36-,38?,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058477

((2R,3aR,7aR)-1-(2-{4-[2-(3,5-Di-tert-butyl-4-hydro...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@H](C[C@H]2CCCC[C@@H]12)C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C43H59F3N4O8S/c1-23(2)34(37(53)43(44,45)46)47-39(55)32-22-27-13-11-12-14-31(27)50(32)40(56)35(24(3)4)48-38(54)26-15-17-28(18-16-26)59(57,58)49-33(51)21-25-19-29(41(5,6)7)36(52)30(20-25)42(8,9)10/h15-20,23-24,27,31-32,34-35,52H,11-14,21-22H2,1-10H3,(H,47,55)(H,48,54)(H,49,51)/t27-,31-,32-,34?,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058471

((2R,3aR,7aR)-1-(2-{4-[(E)-3-(3,5-Di-tert-butyl-4-h...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)\C=C\c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C44H59F3N4O8S/c1-24(2)35(38(54)44(45,46)47)48-40(56)33-23-28-13-11-12-14-32(28)51(33)41(57)36(25(3)4)49-39(55)27-16-18-29(19-17-27)60(58,59)50-34(52)20-15-26-21-30(42(5,6)7)37(53)31(22-26)43(8,9)10/h15-22,24-25,28,32-33,35-36,53H,11-14,23H2,1-10H3,(H,48,56)(H,49,55)(H,50,52)/b20-15+/t28-,32-,33-,35?,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058478

((2R,3aR,7aR)-1-[2-(4-{4-[2-(3,5-Di-tert-butyl-4-hy...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1ccc(CCSc2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)cc1)C(=O)N1[C@H](C[C@H]2CCCC[C@@H]12)C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C50H65F3N4O8S2/c1-28(2)40(43(59)50(51,52)53)54-46(62)39-25-33-13-11-12-14-38(33)57(39)47(63)41(29(3)4)55-44(60)31-19-21-35(22-20-31)67(64,65)56-45(61)32-17-15-30(16-18-32)23-24-66-34-26-36(48(5,6)7)42(58)37(27-34)49(8,9)10/h15-22,26-29,33,38-41,58H,11-14,23-25H2,1-10H3,(H,54,62)(H,55,60)(H,56,61)/t33-,38-,39-,40?,41?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058479
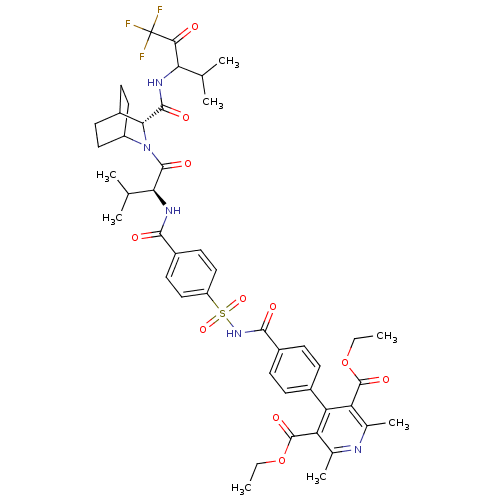
(2,6-Dimethyl-4-[4-(4-{2-methyl-1-[(R)-3-(3,3,3-tri...)Show SMILES CCOC(=O)c1c(C)nc(C)c(C(=O)OCC)c1-c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1C2CCC(CC2)[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F |wU:52.57,wD:39.41,(9.07,-12.21,;7.75,-11.41,;6.43,-12.21,;5.09,-11.41,;5.11,-9.87,;3.77,-12.18,;2.43,-11.41,;2.43,-9.87,;1.08,-12.17,;1.08,-13.72,;-.24,-14.49,;2.43,-14.48,;2.42,-16.03,;3.78,-16.8,;1.08,-16.8,;1.11,-18.34,;-.24,-19.14,;3.74,-13.72,;5.08,-14.49,;6.43,-13.72,;7.72,-14.49,;7.71,-16.03,;6.38,-16.79,;5.08,-16.02,;9.04,-16.83,;10.37,-16.07,;9.02,-18.37,;10.29,-19.29,;11.56,-18.27,;9.65,-20.58,;11.62,-20.06,;12.94,-19.3,;14.27,-20.06,;14.27,-21.64,;12.94,-22.38,;11.6,-21.61,;15.6,-22.41,;15.6,-23.95,;16.93,-21.64,;18.26,-22.41,;18.26,-23.95,;19.36,-24.57,;16.92,-24.72,;19.68,-21.52,;19.87,-19.98,;20.93,-22.42,;20.93,-23.96,;22.32,-24.86,;23.65,-24.11,;23.67,-22.57,;22.13,-22.57,;22.53,-24.06,;22.34,-21.8,;22.5,-20.27,;21.26,-19.36,;24.3,-20.04,;24.46,-18.3,;23.3,-17.27,;23.63,-15.77,;21.84,-17.75,;25.92,-17.83,;26.23,-16.32,;27.06,-18.86,;26.97,-20.46,;28.66,-18.62,;28.12,-19.81,)| Show InChI InChI=1S/C46H54F3N5O11S/c1-9-64-44(60)33-25(7)50-26(8)34(45(61)65-10-2)35(33)27-11-13-30(14-12-27)41(57)53-66(62,63)32-21-17-29(18-22-32)40(56)52-37(24(5)6)43(59)54-31-19-15-28(16-20-31)38(54)42(58)51-36(23(3)4)39(55)46(47,48)49/h11-14,17-18,21-24,28,31,36-38H,9-10,15-16,19-20H2,1-8H3,(H,51,58)(H,52,56)(H,53,57)/t28?,31?,36?,37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058483

((2R,3aR,7aR)-1-(2-{4-[2-(3,5-Di-tert-butyl-4-hydro...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)CSc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@H](C[C@H]2CCCC[C@@H]12)C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C43H59F3N4O8S2/c1-23(2)34(37(53)43(44,45)46)47-39(55)32-19-26-13-11-12-14-31(26)50(32)40(56)35(24(3)4)48-38(54)25-15-17-28(18-16-25)60(57,58)49-33(51)22-59-27-20-29(41(5,6)7)36(52)30(21-27)42(8,9)10/h15-18,20-21,23-24,26,31-32,34-35,52H,11-14,19,22H2,1-10H3,(H,47,55)(H,48,54)(H,49,51)/t26-,31-,32-,34?,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045036

(7-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:50.53,46.49,22.24,wD:11.11,THB:48:50:53.52:55.56,(11.12,-10.02,;9.98,-8.97,;11.14,-7.96,;12.6,-8.45,;13.76,-7.43,;15.21,-7.93,;15.52,-9.44,;14.36,-10.45,;12.9,-9.96,;8.52,-8.48,;8.22,-6.97,;7.37,-9.5,;7.67,-11.01,;9.13,-11.5,;10.29,-10.48,;11.74,-10.98,;12.05,-12.49,;10.89,-13.5,;9.43,-13.01,;5.91,-9,;4.75,-10.02,;5.05,-11.53,;3.29,-9.52,;2.14,-10.54,;2.44,-12.05,;3.84,-12.69,;3.66,-14.22,;4.79,-15.27,;6.26,-14.81,;4.45,-16.77,;5.58,-17.81,;5.25,-19.32,;6.38,-20.36,;6.04,-21.86,;7.17,-22.91,;6.83,-24.41,;2.15,-14.53,;1.36,-15.85,;-.18,-15.83,;-.93,-14.49,;-.15,-13.16,;1.39,-13.18,;2.99,-8.01,;4.15,-7,;5.61,-7.49,;3.85,-5.49,;2.39,-4.99,;2.09,-3.48,;.63,-2.99,;.32,-1.48,;-.53,-4.01,;-1.89,-3.47,;-3.39,-4.13,;-3.19,-5.54,;-1.63,-4.87,;-1.36,-2.93,;-1.82,-1.8,;-.23,-5.52,;1.23,-6.01,;1.53,-7.52,)| Show InChI InChI=1S/C47H57N7O6/c1-52(29-32-16-8-5-9-17-32)46(59)38(26-31-14-6-4-7-15-31)50-44(57)37(27-34-30-53(40-19-12-11-18-36(34)40)42(56)20-10-2-3-13-25-48)49-41(55)28-39-47(60)54-35-23-21-33(22-24-35)43(54)45(58)51-39/h4-9,11-12,14-19,30,33,35,37-39,43H,2-3,10,13,20-29,48H2,1H3,(H,49,55)(H,50,57)(H,51,58)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058494

(3,5-Di-tert-butyl-4-hydroxy-benzoic acid 2,6-di-te...)Show SMILES CC(C)C(NC(=O)c1ccc(cc1)S(=O)(=O)NC(=O)c1cc(c(OC(=O)c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C57H77F3N4O10S/c1-30(2)43(47(66)57(58,59)60)61-50(69)42-29-33-19-17-18-20-41(33)64(42)51(70)44(31(3)4)62-48(67)32-21-23-36(24-22-32)75(72,73)63-49(68)34-25-39(55(11,12)13)46(40(26-34)56(14,15)16)74-52(71)35-27-37(53(5,6)7)45(65)38(28-35)54(8,9)10/h21-28,30-31,33,41-44,65H,17-20,29H2,1-16H3,(H,61,69)(H,62,67)(H,63,68)/t33-,41-,42-,43?,44?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058490

((2R,3aR,7aR)-1-(2-{4-Chloro-3-[2-(3,5-di-tert-buty...)Show SMILES CC(C)C(NC(=O)c1ccc(Cl)c(c1)S(=O)(=O)NC(=O)CSc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)C(=O)N1[C@@H]2CCCC[C@@H]2C[C@@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C43H58ClF3N4O8S2/c1-22(2)34(37(54)43(45,46)47)48-39(56)31-17-24-13-11-12-14-30(24)51(31)40(57)35(23(3)4)49-38(55)25-15-16-29(44)32(18-25)61(58,59)50-33(52)21-60-26-19-27(41(5,6)7)36(53)28(20-26)42(8,9)10/h15-16,18-20,22-24,30-31,34-35,53H,11-14,17,21H2,1-10H3,(H,48,56)(H,49,55)(H,50,52)/t24-,30-,31-,34?,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045039

(6-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)OCCCCCCN)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:51.54,47.50,22.24,wD:11.12,THB:49:51:54.53:56.57,(11.28,-9.71,;10.14,-8.66,;11.3,-7.64,;12.76,-8.14,;13.92,-7.12,;15.38,-7.61,;15.68,-9.13,;14.52,-10.14,;13.06,-9.65,;8.69,-8.17,;8.38,-6.66,;7.53,-9.18,;7.83,-10.69,;9.29,-11.19,;10.45,-10.17,;11.91,-10.66,;12.21,-12.17,;11.05,-13.19,;9.59,-12.7,;6.07,-8.69,;4.91,-9.71,;5.22,-11.22,;3.46,-9.21,;2.3,-10.23,;2.6,-11.74,;4,-12.38,;3.82,-13.91,;4.95,-14.96,;6.42,-14.5,;4.61,-16.46,;5.75,-17.5,;5.41,-19.01,;6.54,-20.05,;6.2,-21.55,;7.33,-22.6,;6.99,-24.1,;8.13,-25.14,;2.31,-14.22,;1.52,-15.54,;-.02,-15.52,;-.77,-14.18,;.02,-12.85,;1.56,-12.87,;3.15,-7.7,;4.31,-6.69,;5.77,-7.18,;4.01,-5.18,;2.55,-4.68,;2.25,-3.17,;.79,-2.68,;.49,-1.17,;-.37,-3.7,;-1.73,-3.16,;-3.23,-3.82,;-3.03,-5.23,;-1.46,-4.56,;-1.2,-2.62,;-1.66,-1.49,;-.07,-5.21,;1.39,-5.7,;1.69,-7.21,)| Show InChI InChI=1S/C47H57N7O7/c1-52(29-32-16-8-5-9-17-32)45(58)38(26-31-14-6-4-7-15-31)50-43(56)37(49-41(55)28-39-46(59)54-35-22-20-33(21-23-35)42(54)44(57)51-39)27-34-30-53(40-19-11-10-18-36(34)40)47(60)61-25-13-3-2-12-24-48/h4-11,14-19,30,33,35,37-39,42H,2-3,12-13,20-29,48H2,1H3,(H,49,55)(H,50,56)(H,51,57)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045040

(CHEMBL3144343 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:43.46,11.11,39.42,22.24,THB:41:43:46.45:48.49,(10.33,-13,;9.17,-14.02,;9.47,-15.53,;10.93,-16.02,;11.23,-17.53,;12.69,-18.03,;13.85,-17.01,;13.55,-15.5,;12.09,-15.01,;7.71,-13.52,;6.56,-14.54,;7.41,-12.01,;8.57,-11,;8.26,-9.49,;9.42,-8.47,;9.12,-6.96,;7.66,-6.47,;6.5,-7.48,;6.81,-8.99,;5.95,-11.52,;5.65,-10.01,;5.99,-8.51,;4.19,-9.52,;3.03,-10.53,;3.34,-12.04,;4.73,-12.69,;4.56,-14.22,;5.69,-15.26,;5.35,-16.76,;3.05,-14.52,;2.26,-15.84,;.72,-15.82,;-.03,-14.48,;.75,-13.16,;2.29,-13.18,;3.89,-8.01,;2.43,-7.51,;1.27,-8.53,;2.13,-6,;.67,-5.51,;.37,-4,;-1.09,-3.51,;-1.39,-2,;-2.25,-4.52,;-3.61,-3.99,;-5.11,-4.65,;-4.91,-6.06,;-3.34,-5.39,;-3.08,-3.45,;-3.54,-2.32,;-1.95,-6.03,;-.49,-6.53,;-.19,-8.04,)| Show InChI InChI=1S/C41H44N6O6/c1-45(23-27-12-6-3-7-13-27)40(52)33(20-26-10-4-2-5-11-26)43-38(50)32(21-29-24-46(25-48)35-15-9-8-14-31(29)35)42-36(49)22-34-41(53)47-30-18-16-28(17-19-30)37(47)39(51)44-34/h2-15,24-25,28,30,32-34,37H,16-23H2,1H3,(H,42,49)(H,43,50)(H,44,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058476

((S)-1-[(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)C(NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CSc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C46H56ClF3N4O8S2/c1-25(2)37(39(56)46(48,49)50)52-42(59)36-21-28-11-9-10-12-35(28)54(36)43(60)34(24-63-30-22-32(44(3,4)5)38(55)33(23-30)45(6,7)8)51-40(57)26-13-15-27(16-14-26)41(58)53-64(61,62)31-19-17-29(47)18-20-31/h13-20,22-23,25,28,34-37,55H,9-12,21,24H2,1-8H3,(H,51,57)(H,52,59)(H,53,58)/t28?,34-,35?,36+,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50045037
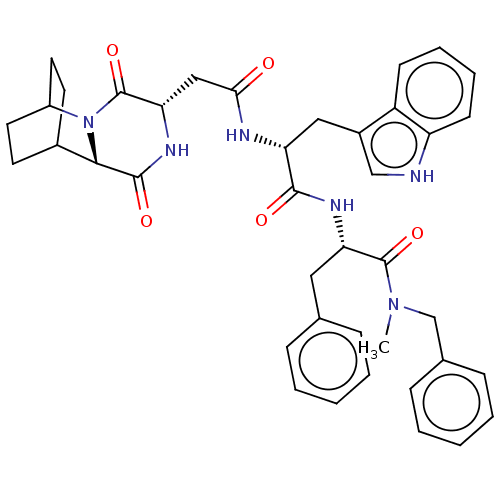
(CHEMBL3144352 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:41.44,37.40,wD:11.11,22.24,THB:39:41:44.43:46.47,(10.7,-11.55,;10.4,-10.04,;11.55,-9.02,;13.01,-9.52,;14.17,-8.5,;15.63,-8.99,;15.93,-10.5,;14.77,-11.52,;13.31,-11.03,;8.94,-9.55,;8.63,-8.04,;7.78,-10.56,;8.08,-12.07,;6.93,-13.09,;7.23,-14.6,;6.07,-15.62,;4.61,-15.12,;4.31,-13.61,;5.47,-12.6,;6.32,-10.07,;5.16,-11.09,;4.52,-12.48,;3.71,-10.59,;3.4,-9.08,;4.56,-8.07,;6.06,-8.4,;6.85,-7.08,;5.83,-5.92,;6.01,-4.39,;4.78,-3.47,;3.36,-4.08,;3.18,-5.61,;4.42,-6.53,;2.55,-11.61,;1.09,-11.12,;.79,-9.61,;-.07,-12.13,;-1.53,-11.64,;-1.83,-10.13,;-3.29,-9.63,;-3.59,-8.12,;-4.44,-10.65,;-5.8,-10.12,;-7.3,-10.77,;-7.1,-12.18,;-5.54,-11.51,;-5.28,-9.58,;-5.73,-8.44,;-4.14,-12.16,;-2.68,-12.66,;-2.38,-14.17,)| Show InChI InChI=1S/C40H44N6O5/c1-45(24-26-12-6-3-7-13-26)39(50)33(20-25-10-4-2-5-11-25)43-37(48)32(21-28-23-41-31-15-9-8-14-30(28)31)42-35(47)22-34-40(51)46-29-18-16-27(17-19-29)36(46)38(49)44-34/h2-15,23,27,29,32-34,36,41H,16-22,24H2,1H3,(H,42,47)(H,43,48)(H,44,49) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against rat NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045037

(CHEMBL3144352 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:41.44,37.40,wD:11.11,22.24,THB:39:41:44.43:46.47,(10.7,-11.55,;10.4,-10.04,;11.55,-9.02,;13.01,-9.52,;14.17,-8.5,;15.63,-8.99,;15.93,-10.5,;14.77,-11.52,;13.31,-11.03,;8.94,-9.55,;8.63,-8.04,;7.78,-10.56,;8.08,-12.07,;6.93,-13.09,;7.23,-14.6,;6.07,-15.62,;4.61,-15.12,;4.31,-13.61,;5.47,-12.6,;6.32,-10.07,;5.16,-11.09,;4.52,-12.48,;3.71,-10.59,;3.4,-9.08,;4.56,-8.07,;6.06,-8.4,;6.85,-7.08,;5.83,-5.92,;6.01,-4.39,;4.78,-3.47,;3.36,-4.08,;3.18,-5.61,;4.42,-6.53,;2.55,-11.61,;1.09,-11.12,;.79,-9.61,;-.07,-12.13,;-1.53,-11.64,;-1.83,-10.13,;-3.29,-9.63,;-3.59,-8.12,;-4.44,-10.65,;-5.8,-10.12,;-7.3,-10.77,;-7.1,-12.18,;-5.54,-11.51,;-5.28,-9.58,;-5.73,-8.44,;-4.14,-12.16,;-2.68,-12.66,;-2.38,-14.17,)| Show InChI InChI=1S/C40H44N6O5/c1-45(24-26-12-6-3-7-13-26)39(50)33(20-25-10-4-2-5-11-25)43-37(48)32(21-28-23-41-31-15-9-8-14-30(28)31)42-35(47)22-34-40(51)46-29-18-16-27(17-19-29)36(46)38(49)44-34/h2-15,23,27,29,32-34,36,41H,16-22,24H2,1H3,(H,42,47)(H,43,48)(H,44,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058482

((S)-1-{(R)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)C(NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CCCCNC(=O)Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C51H65ClF3N5O9S/c1-29(2)42(44(63)51(53,54)55)58-47(66)40-28-33-13-9-10-15-39(33)60(40)48(67)38(14-11-12-24-56-41(61)27-30-25-36(49(3,4)5)43(62)37(26-30)50(6,7)8)57-45(64)31-16-18-32(19-17-31)46(65)59-70(68,69)35-22-20-34(52)21-23-35/h16-23,25-26,29,33,38-40,42,62H,9-15,24,27-28H2,1-8H3,(H,56,61)(H,57,64)(H,58,66)(H,59,65)/t33?,38-,39?,40+,42?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045032
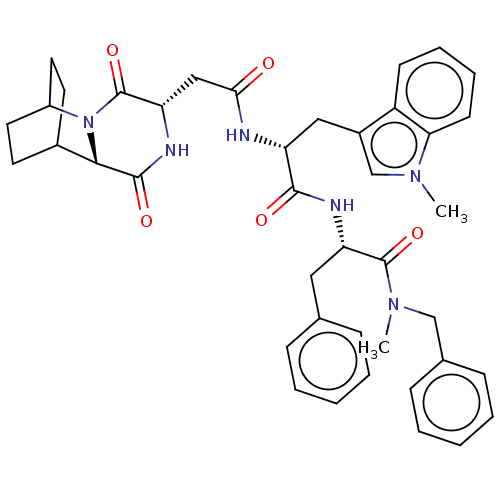
(CHEMBL3144353 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:42.45,38.41,wD:22.24,11.12,THB:40:42:45.44:47.48,(10.71,-11.65,;10.41,-10.14,;11.57,-9.13,;13.03,-9.62,;14.18,-8.6,;15.64,-9.1,;15.94,-10.61,;14.79,-11.62,;13.33,-11.13,;8.95,-9.65,;8.65,-8.14,;7.8,-10.67,;8.1,-12.18,;6.94,-13.19,;7.24,-14.7,;6.09,-15.72,;4.63,-15.23,;4.33,-13.72,;5.48,-12.7,;6.34,-10.17,;5.18,-11.19,;4.54,-12.59,;3.72,-10.7,;3.42,-9.19,;4.58,-8.17,;6.08,-8.51,;6.87,-7.18,;8.4,-7.04,;5.85,-6.03,;6.03,-4.5,;4.79,-3.58,;3.38,-4.19,;3.2,-5.72,;4.43,-6.64,;2.56,-11.71,;1.11,-11.22,;.8,-9.71,;-.05,-12.24,;-1.51,-11.74,;-1.81,-10.23,;-3.27,-9.74,;-3.57,-8.23,;-4.43,-10.76,;-5.79,-10.22,;-7.29,-10.88,;-7.09,-12.29,;-5.52,-11.62,;-5.26,-9.68,;-5.72,-8.55,;-4.12,-12.27,;-2.67,-12.76,;-2.36,-14.27,)| Show InChI InChI=1S/C41H46N6O5/c1-45-25-29(31-15-9-10-16-35(31)45)22-32(42-36(48)23-34-41(52)47-30-19-17-28(18-20-30)37(47)39(50)44-34)38(49)43-33(21-26-11-5-3-6-12-26)40(51)46(2)24-27-13-7-4-8-14-27/h3-16,25,28,30,32-34,37H,17-24H2,1-2H3,(H,42,48)(H,43,49)(H,44,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058488
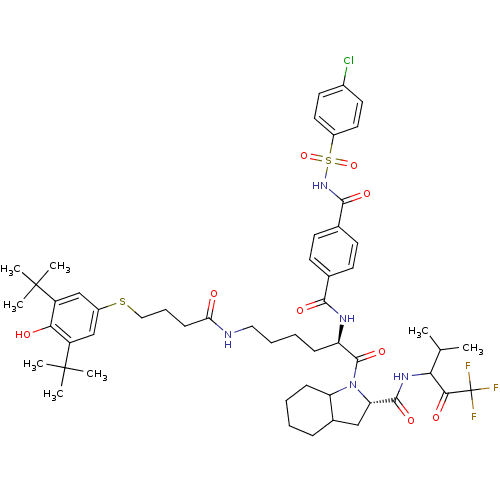
((S)-1-{(R)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)C(NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CCCCNC(=O)CCCSc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C53H69ClF3N5O9S2/c1-31(2)44(46(65)53(55,56)57)60-49(68)42-28-34-14-9-10-16-41(34)62(42)50(69)40(59-47(66)32-18-20-33(21-19-32)48(67)61-73(70,71)37-24-22-35(54)23-25-37)15-11-12-26-58-43(63)17-13-27-72-36-29-38(51(3,4)5)45(64)39(30-36)52(6,7)8/h18-25,29-31,34,40-42,44,64H,9-17,26-28H2,1-8H3,(H,58,63)(H,59,66)(H,60,68)(H,61,67)/t34?,40-,41?,42+,44?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Leukocyte Elastase from human sputum |
J Med Chem 40: 1906-18 (1997)
Article DOI: 10.1021/jm960772z
BindingDB Entry DOI: 10.7270/Q2MS3RW1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045035
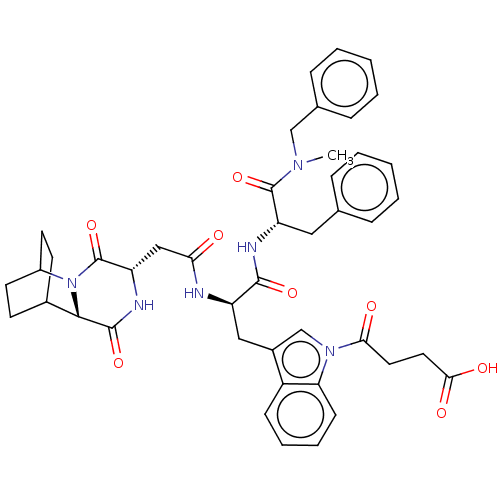
(4-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCC(O)=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:48.51,11.11,44.47,wD:22.24,THB:46:48:51.50:53.54,(10.95,-12.84,;9.79,-13.85,;10.1,-15.36,;11.55,-15.86,;11.86,-17.37,;13.32,-17.86,;14.47,-16.84,;14.17,-15.33,;12.71,-14.84,;8.33,-13.36,;7.18,-14.38,;8.03,-11.85,;9.19,-10.83,;8.89,-9.32,;10.04,-8.31,;9.74,-6.8,;8.28,-6.3,;7.13,-7.32,;7.43,-8.83,;6.49,-11.83,;5.74,-10.49,;6.52,-9.16,;4.2,-10.47,;3.45,-9.13,;4.23,-7.8,;5.76,-7.66,;6.1,-6.16,;7.52,-5.55,;8.98,-6.04,;7.7,-4.02,;9.11,-3.41,;9.29,-1.88,;8.06,-.96,;10.7,-1.27,;4.78,-5.37,;4.48,-3.86,;3.02,-3.37,;1.86,-4.38,;2.16,-5.89,;3.62,-6.39,;3.41,-11.79,;1.87,-11.78,;1.12,-10.43,;1.09,-13.1,;-.45,-13.08,;-1.21,-11.74,;-2.75,-11.72,;-3.5,-10.38,;-3.53,-13.04,;-4.99,-12.96,;-6.22,-14.05,;-5.59,-15.32,;-4.31,-14.2,;-4.66,-12.28,;-5.44,-11.34,;-2.78,-14.39,;-1.24,-14.41,;-.48,-15.75,)| Show InChI InChI=1S/C44H48N6O8/c1-48(25-28-12-6-3-7-13-28)43(57)34(22-27-10-4-2-5-11-27)46-41(55)33(23-30-26-49(38(52)20-21-39(53)54)36-15-9-8-14-32(30)36)45-37(51)24-35-44(58)50-31-18-16-29(17-19-31)40(50)42(56)47-35/h2-15,26,29,31,33-35,40H,16-25H2,1H3,(H,45,51)(H,46,55)(H,47,56)(H,53,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045033

(1N-[1-[1-benzyl(methyl)carbamoyl-2-phenyl-(1S)-eth...)Show SMILES CC(O)[C@H](NC(C)=O)C(=O)N[C@H](Cc1cn(C=O)c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C35H39N5O6/c1-23(42)32(36-24(2)43)34(45)37-29(19-27-21-40(22-41)31-17-11-10-16-28(27)31)33(44)38-30(18-25-12-6-4-7-13-25)35(46)39(3)20-26-14-8-5-9-15-26/h4-17,21-23,29-30,32,42H,18-20H2,1-3H3,(H,36,43)(H,37,45)(H,38,44)/t23?,29-,30+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50199883
(3,4‐Dichloroisocoumarin (2) | 3,4-Dichloro-i...)Show InChI InChI=1S/C9H4Cl2O2/c10-7-5-3-1-2-4-6(5)9(12)13-8(7)11/h1-4H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for the inhibition of human leukocyte elastase(HLE) |
Bioorg Med Chem Lett 3: 2547-2552 (1993)
Article DOI: 10.1016/S0960-894X(01)80714-9
BindingDB Entry DOI: 10.7270/Q2CV4HNW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50045032

(CHEMBL3144353 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:42.45,38.41,wD:22.24,11.12,THB:40:42:45.44:47.48,(10.71,-11.65,;10.41,-10.14,;11.57,-9.13,;13.03,-9.62,;14.18,-8.6,;15.64,-9.1,;15.94,-10.61,;14.79,-11.62,;13.33,-11.13,;8.95,-9.65,;8.65,-8.14,;7.8,-10.67,;8.1,-12.18,;6.94,-13.19,;7.24,-14.7,;6.09,-15.72,;4.63,-15.23,;4.33,-13.72,;5.48,-12.7,;6.34,-10.17,;5.18,-11.19,;4.54,-12.59,;3.72,-10.7,;3.42,-9.19,;4.58,-8.17,;6.08,-8.51,;6.87,-7.18,;8.4,-7.04,;5.85,-6.03,;6.03,-4.5,;4.79,-3.58,;3.38,-4.19,;3.2,-5.72,;4.43,-6.64,;2.56,-11.71,;1.11,-11.22,;.8,-9.71,;-.05,-12.24,;-1.51,-11.74,;-1.81,-10.23,;-3.27,-9.74,;-3.57,-8.23,;-4.43,-10.76,;-5.79,-10.22,;-7.29,-10.88,;-7.09,-12.29,;-5.52,-11.62,;-5.26,-9.68,;-5.72,-8.55,;-4.12,-12.27,;-2.67,-12.76,;-2.36,-14.27,)| Show InChI InChI=1S/C41H46N6O5/c1-45-25-29(31-15-9-10-16-35(31)45)22-32(42-36(48)23-34-41(52)47-30-19-17-28(18-20-30)37(47)39(50)44-34)38(49)43-33(21-26-11-5-3-6-12-26)40(51)46(2)24-27-13-7-4-8-14-27/h3-16,25,28,30,32-34,37H,17-24H2,1-2H3,(H,42,48)(H,43,49)(H,44,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for antagonist activityt against NK-2 receptor in rabbit pulmonary artery by using Neurokinin A as agonist |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50045035

(4-(3-{2-[1-(Benzyl-methyl-carbamoyl)-2-phenyl-ethy...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C(=O)CCC(O)=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:48.51,11.11,44.47,wD:22.24,THB:46:48:51.50:53.54,(10.95,-12.84,;9.79,-13.85,;10.1,-15.36,;11.55,-15.86,;11.86,-17.37,;13.32,-17.86,;14.47,-16.84,;14.17,-15.33,;12.71,-14.84,;8.33,-13.36,;7.18,-14.38,;8.03,-11.85,;9.19,-10.83,;8.89,-9.32,;10.04,-8.31,;9.74,-6.8,;8.28,-6.3,;7.13,-7.32,;7.43,-8.83,;6.49,-11.83,;5.74,-10.49,;6.52,-9.16,;4.2,-10.47,;3.45,-9.13,;4.23,-7.8,;5.76,-7.66,;6.1,-6.16,;7.52,-5.55,;8.98,-6.04,;7.7,-4.02,;9.11,-3.41,;9.29,-1.88,;8.06,-.96,;10.7,-1.27,;4.78,-5.37,;4.48,-3.86,;3.02,-3.37,;1.86,-4.38,;2.16,-5.89,;3.62,-6.39,;3.41,-11.79,;1.87,-11.78,;1.12,-10.43,;1.09,-13.1,;-.45,-13.08,;-1.21,-11.74,;-2.75,-11.72,;-3.5,-10.38,;-3.53,-13.04,;-4.99,-12.96,;-6.22,-14.05,;-5.59,-15.32,;-4.31,-14.2,;-4.66,-12.28,;-5.44,-11.34,;-2.78,-14.39,;-1.24,-14.41,;-.48,-15.75,)| Show InChI InChI=1S/C44H48N6O8/c1-48(25-28-12-6-3-7-13-28)43(57)34(22-27-10-4-2-5-11-27)46-41(55)33(23-30-26-49(38(52)20-21-39(53)54)36-15-9-8-14-32(30)36)45-37(51)24-35-44(58)50-31-18-16-29(17-19-31)40(50)42(56)47-35/h2-15,26,29,31,33-35,40H,16-25H2,1H3,(H,45,51)(H,46,55)(H,47,56)(H,53,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human NK-2 receptor |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50045040

(CHEMBL3144343 | N-Benzyl-2-[2-[2-(3,6-dioxo-2,5-di...)Show SMILES CN(Cc1ccccc1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cn(C=O)c2ccccc12)NC(=O)C[C@@H]1NC(=O)[C@@H]2C3CCC(CC3)N2C1=O |r,wU:43.46,11.11,39.42,22.24,THB:41:43:46.45:48.49,(10.33,-13,;9.17,-14.02,;9.47,-15.53,;10.93,-16.02,;11.23,-17.53,;12.69,-18.03,;13.85,-17.01,;13.55,-15.5,;12.09,-15.01,;7.71,-13.52,;6.56,-14.54,;7.41,-12.01,;8.57,-11,;8.26,-9.49,;9.42,-8.47,;9.12,-6.96,;7.66,-6.47,;6.5,-7.48,;6.81,-8.99,;5.95,-11.52,;5.65,-10.01,;5.99,-8.51,;4.19,-9.52,;3.03,-10.53,;3.34,-12.04,;4.73,-12.69,;4.56,-14.22,;5.69,-15.26,;5.35,-16.76,;3.05,-14.52,;2.26,-15.84,;.72,-15.82,;-.03,-14.48,;.75,-13.16,;2.29,-13.18,;3.89,-8.01,;2.43,-7.51,;1.27,-8.53,;2.13,-6,;.67,-5.51,;.37,-4,;-1.09,-3.51,;-1.39,-2,;-2.25,-4.52,;-3.61,-3.99,;-5.11,-4.65,;-4.91,-6.06,;-3.34,-5.39,;-3.08,-3.45,;-3.54,-2.32,;-1.95,-6.03,;-.49,-6.53,;-.19,-8.04,)| Show InChI InChI=1S/C41H44N6O6/c1-45(23-27-12-6-3-7-13-27)40(52)33(20-26-10-4-2-5-11-26)43-38(50)32(21-29-24-46(25-48)35-15-9-8-14-31(29)35)42-36(49)22-34-41(53)47-30-18-16-28(17-19-30)37(47)39(51)44-34/h2-15,24-25,28,30,32-34,37H,16-23H2,1H3,(H,42,49)(H,43,50)(H,44,51) | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Tested for binding affinity against rat NK-2 receptor transfected on CHO cells using [125I]-His] NKA as radioligand |
J Med Chem 36: 1654-61 (1993)
BindingDB Entry DOI: 10.7270/Q25M66BM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































